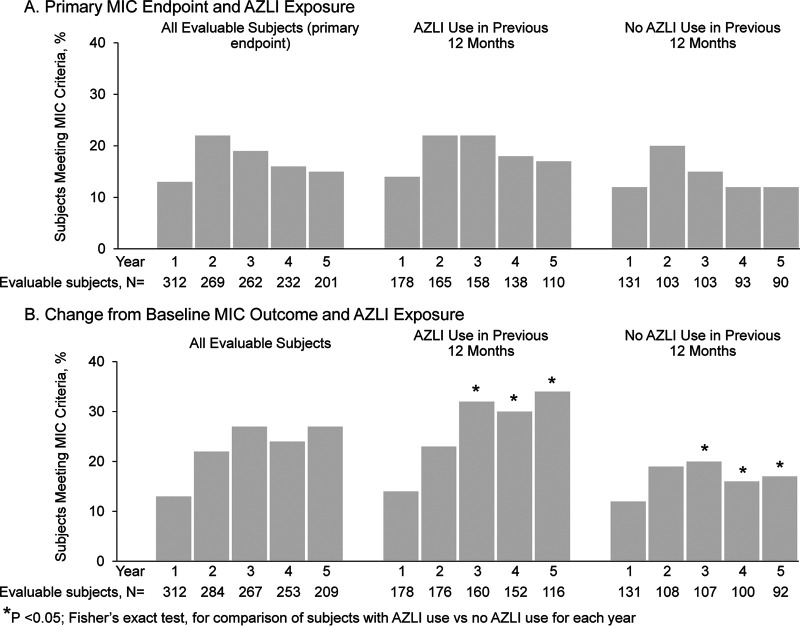
FIG 1.
Changes in aztreonam susceptibility of Pseudomonas aeruginosa isolates. (A) Proportions of evaluable subjects who met the primary endpoint overall and by AZLI use in the previous 12 months (yes/no). The primary endpoint was met in a reporting year if the aztreonam MIC for the least susceptible P. aeruginosa isolate for that year was >8 μg/ml and had increased ≥4-fold compared with the MIC for the least susceptible isolate from the previous reporting year. (B) Proportions of evaluable subjects who met the secondary microbiologic outcome overall and by AZLI use in the previous 12 months (yes/no). The secondary microbiologic outcome was met in a reporting year if the aztreonam MIC for the least susceptible P. aeruginosa isolate for that year was >8 μg/ml and had increased ≥4-fold compared with the MIC for the least susceptible isolate observed at the baseline year. Data regarding AZLI use were not available for all subjects for either assessment; therefore, the numbers of evaluable subjects with and without AZLI use are usually slightly smaller than the total number of evaluable subjects. Note that subjects could move between AZLI use categories (yes/no for each year) across the study. P values were determined with Fisher’s exact test and are presented for descriptive purposes only.