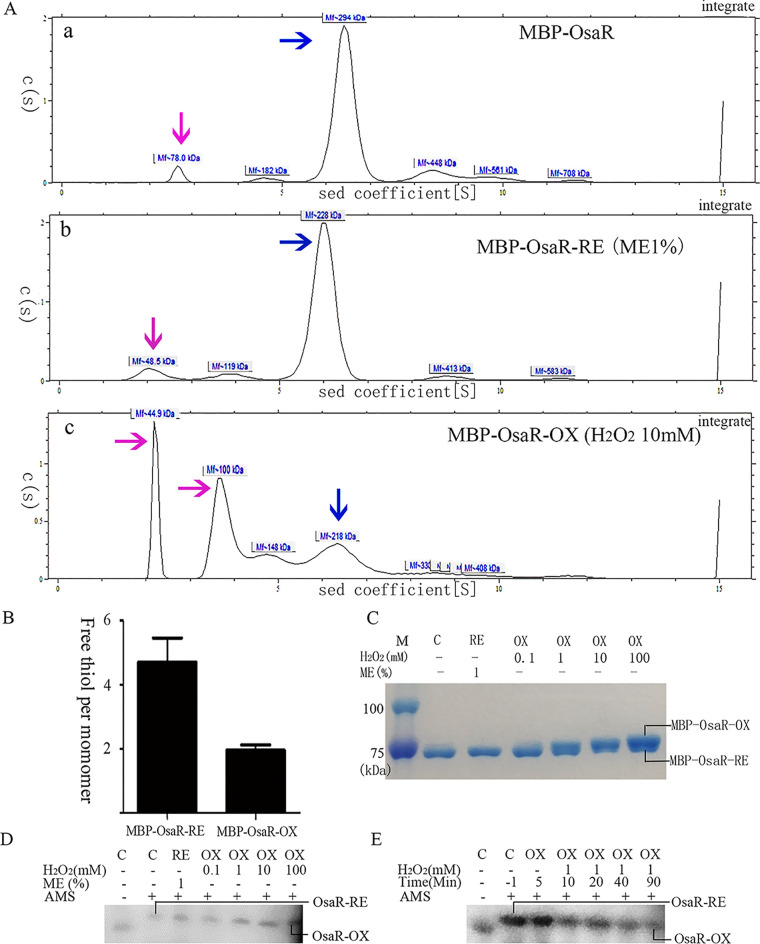
FIG 6.
Change of OsaR protein redox state with H2O2 treatment. Purified MBP-OsaR (40 μM) was incubated with 1% ME for 20 min to reduce MBP-OsaR (MBP-OsaR-RE) or with 10 mM H2O2 for 20 min to obtain oxidized MBP-OsaR (MBP-OsaR-OX). (A) Analytical ultracentrifugation was employed to detect the state of MBP-OsaR aggregation. The blue arrow indicates the polymer, and the pink arrow indicates the MBP-OsaR monomer or MBP broken away from MBP-OsaR. OsaR aggregates into pellets due to its instability and cannot be shown on the curve. (B) Measurement of free thiol contents in MBP-OsaR-RE and MBP-OsaR-OX (MBP has no cysteine residues). (C to E) Nonreducing SDS-PAGE (8%) of MBP-OsaR (2 μM) reduced with ME (1%) or oxidized with the indicated concentration of H2O2 in vitro. A total of 225 μg of protein was loaded per lane. Protein mobility was visualized using Coomassie brilliant blue staining. PAK WT bacteria containing osaR-His fusion were treated with 0, 0.1, 1, 10, or 100 mM H2O2 for 20 min (D) or the strain was treated with 1 mM H2O2 every 10 min (E) and then aliquots were taken at the given time points. After that, the samples were precipitated with TCA and then treated with AMS. Again, separation and detection of OsaR-His were achieved by nonreducing SDS-PAGE (10%) and immunoblotting. OsaR-RE or RE, the reduced form; OsaR-OX or OX, the oxidized form; C, the absence of ME or H2O2. All data were obtained from at least three independent experiments with at least three replicates.