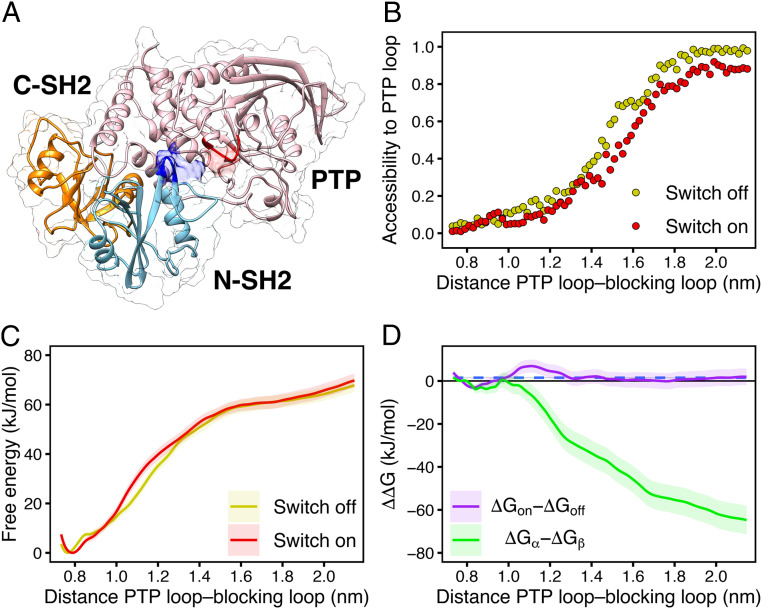
Fig. 6.
(A) Exemplary open and active structure of SHP2 as obtained by pulling simulation. The N-SH2 domain (cyan cartoon) moved from its position in the autoinhibited state to a different position relative to the PTP (pink) and C-SH2 domains (orange), leaving the catalytic site (red) solvent exposed. (B) Accessibility of the PTP catalytic site versus the distance between the blocking loop and the catalytic PTP loop used as reaction coordinate in pulling simulation, defined as the backbone centers-of-mass distance between residues 60 to 62 and residues 460 to 462. The N-SH2 binding cleft was restrained either in closed (switch off, yellow dots) or in open (switch on, red dots) conformation. The accessibility of the PTP catalytic site was defined as the ratio of the SAS areas of the PTP loop, obtained including or excluding the N-SH2 domain. The probe radius was 2.38 Å corresponding to a phosphate ion. (C) PMFs of SHP2 opening with the N-SH2 binding cleft restrained either in closed (switch off, yellow line) or in open (switch on, red line) conformation. (D) profiles of SHP2 opening, calculated from the difference of the PMFs for “switch on” relative to the “switch off” conformation in C (violet line), and, for comparison, for the -state relative to the -state (green line), calculated from PMFs in ref. (43). Dashed blue line represents the average G value of the ΔGon − ΔGoff profile.