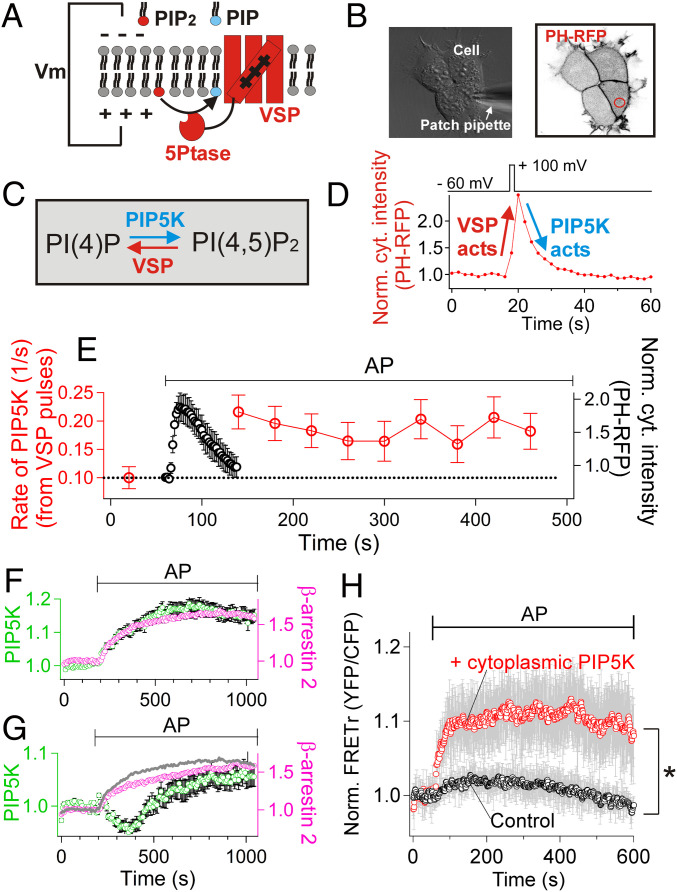
Fig. 4.
PAR2 activates PIP5K. (A) Cartoon of voltage-sensitive 5-phosphatase (VSP) used to deplete PM PI(4,5)P2. (B) Differential interference contrast (DIC) and confocal imaging combined with patch clamp. PAR2-CFP, GFP-VSP and PH-RFP were expressed at the same time. (C) Reaction scheme for PM PI(4,5)P2 depletion by VSP and resynthesis by PIP5K. (D) Time course of cytoplasmic PH-RFP during and after one brief activation of VSP by a voltage jump. A representative trace. (E) Red symbols: Catalytic rate constant of PIP5K determined from the exponential recovery of PI(4,5)P2 after repeated VSP pulses applied before and during treatment with AP (100 μM). Mean of five independent experiments. Black symbols: mean time course of PH-RFP probe translocation during the activation of PAR2 by AP. (F and G) Average intensity of clustered β-arrestin-2-mRFP and of GFP-PIP5K-Iγ recorded in conventional TIRF microscopy while AP was applied to PAR2-expressing cells. In the analysis, regions of interest (ROIs) containing individual β-arrestin-2-mRFP clusters (magenta) were selected to measure intensity changes of GFP-PIP5K-Iγ (green), reflecting recruitment of the kinase into β-arrestin 2 clusters. (F) One subset of data with monotonic PIP5K-Iγ recruitment into β-arrestin 2 clusters (group 1, 14 ROIs). (G) The second, remaining subset of data with biphasic PIP5K-Iγ signals at β-arrestin 2 clusters (group 2, 13 ROIs). The gray line was copied from the β-arrestin 2 clustering in F for comparison. The gray line and magenta symbols are significantly different (P < 0.001). (H) FRET between CFP-tagged cytoplasmic PIP5K-Iγ and YFP-tagged β-arrestin 2 (“+ cytoplasmic PIP5K,” n = 9 cells). For the control experiments (“Control,” n = 8 cells), cells were transfected with CFP instead of CFP-PIP5K-Iγ together with β-arrestin-2-YFP and dark PAR2. AP (100 µM) was used to activate PAR2 receptors. *P < 0.05.