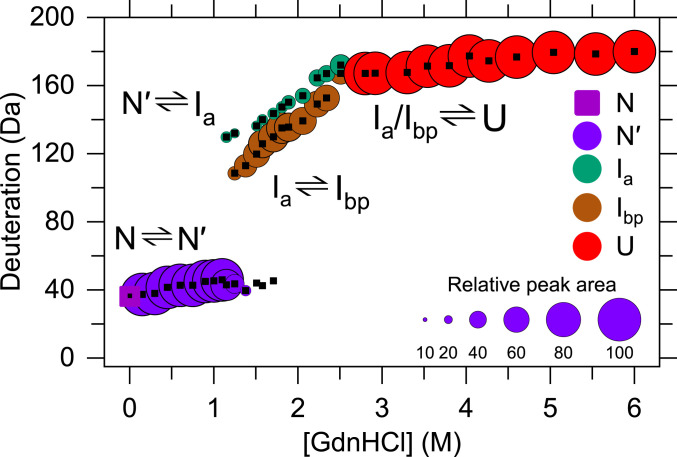
Fig. 3.
The deuterium uptake and relative peak areas of the +28-charge state are shown for species in the TmIGPS folding mechanism from equilibrium HDX-MS experiments on the intact protein after equilibration for 9 d in different GdnHCl concentrations. Gaussian fitting was used to determine the deuterium uptake (black dots) and the relative peak area (circles) for intermediates in the +28-charge state (Fig. 2). The maximum number of exchangeable NHs, 212 (222 residues, 9 prolines) sets an upper limit on the deuterium uptake. The uptake of 181 deuteriums in the unfolded control represent 15% back exchange with hydrogen during the workflow (SI Appendix, Table S2).