Fig. 1.
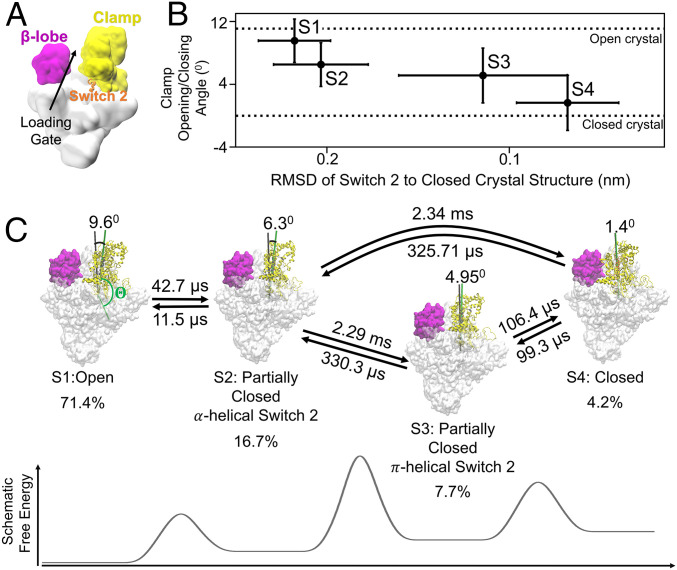
qMSM identifies four metastable states corresponding to the opening and closing of RNAP clamp domain. (A) The overall cartoon representation of bacterial holoenzyme and its domains, clamp, β-lobe, and Switch 2 region are shown as yellow, magenta, and orange, respectively. (B) Four macrostates of the clamp domain of RNAP are identified by MSM. The metastable states are represented as clamp opening/closing angles and rmsd of Switch 2 region (β’611 to 620) aligned to the Switch 2 region of the closed clamp in the crystal structure. The clamp opening/closing angle () is defined as the torsion angle between four centers of mass of C-α of residues 1) ω 60 to 68, 2) β’1461 to 1468, 3) β1031 to 1033 and β’620 to 622, and 4) β’568 to 573 and is offset by the angle of closed clamp crystal structure (). (C) The transition time between metastable states and the stationary population of each state is shown. The transitions between S1 and S2, as well as S3 and S4, are fast. The transitions between S1 or S2 to either S3 or S4 are slow, as indicated by the higher free-energy barrier. The full list of transition times is shown in SI Appendix, Table S1.