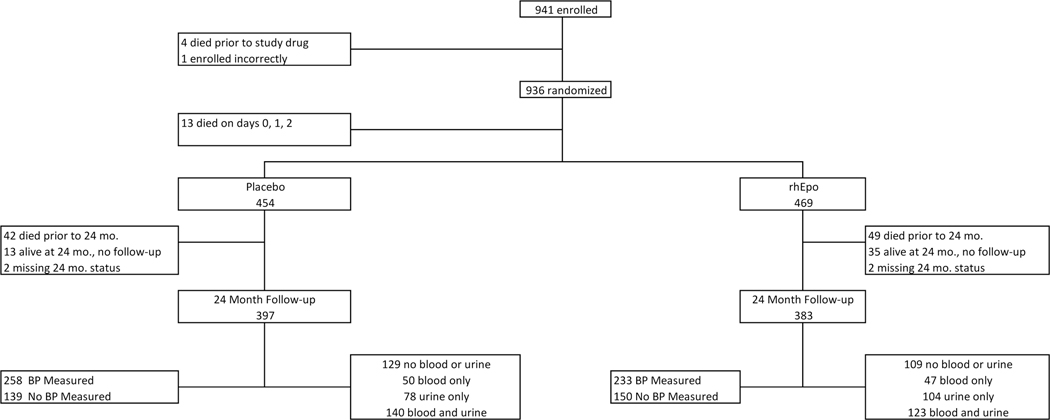
Figure 1 (online only):
941 subjects were enrolled in the PENUT study. Of the 941, 18 were excluded from this study as 4 died prior to study drug, 1 was enrolled incorrectly and 13 died on days 0,1, 2 and we could not assign any kidney related outcomes. Therefore, the final sample of participants for short-term outcomes in REPaIReD were the 923 who received study drug and were alive on day 3. Of the 923, 454 received placebo and 469 received rhEpo. At the 24 month follow-up, 780 infants were evaluated (397 in placebo and 383 in rhEpo) groups. The number who had blood/urine collected at the 24 month visit are described in the figure and in text.