Fig. 7. Mechanism by which pleckstrin-2 supports proliferation, decreases apoptosis, and enhances enucleation in ineffective erythropoiesis.
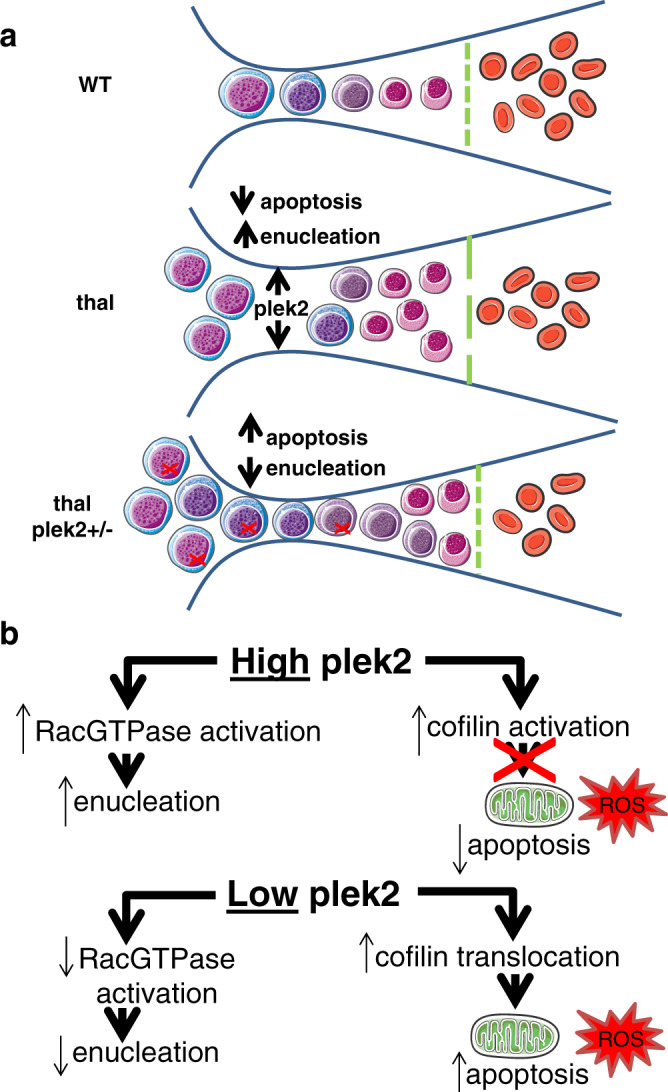
a The normal balance of differentiation and proliferation enables effective erythropoiesis in WT mouse bone marrow. In β-thalassemic bone marrows, ineffective erythropoiesis results from an imbalance, with relatively more proliferating erythroblasts that differentiate and enucleate to form mature RBCs. When ROS are increased in β-thalassemic erythroblasts, elevated Epo enables multiple downstream mechanisms. Plek2 serves as a mechanism downstream of Epo by which more β-thalassemic erythroblasts evade apoptosis and increase erythroblast enucleation (green line). In thal plek2+/− bone marrow, more erythroblasts undergo apoptosis (red X) and fewer enucleate (green line), resulting in worsening anemia compared with β-thalassemic mice. b When membrane-associated pleckstrin-2 is increased, it’s binding to cofilin and consequent prevention of cofilin translocation to the mitochondria results in decreased apoptosis despite elevation of ROS in β-thalassemic erythroblasts. In addition, interaction with and activation of Rac1 results in enhanced enucleation in high-pleckstrin-2 states. Thus, when membrane-associated pleckstrin-2 is decreased in the setting of increased erythroblast ROS, relatively more cofilin translocation to the mitochondria leads to apoptosis and decreased Rac1 activation results in relatively decreased enucleation, leading to worsening ineffective erythropoiesis in β-thalassemia. WT = wild type; thal = β-thalassemia; plek2 = pleckstrin-2; ROS = reactive oxygen species.
