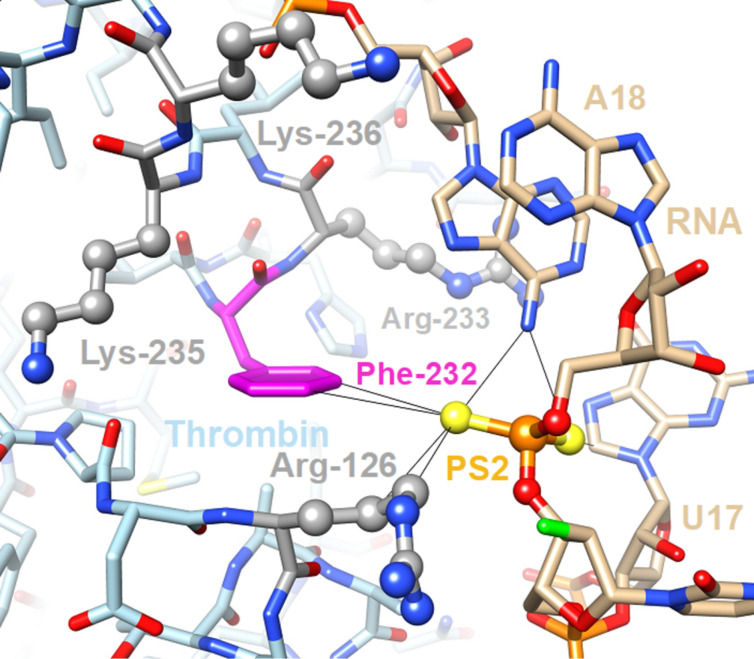
Figure 3.
Close-up view of a key interaction between the PS2-modified antithrombin RNA aptamer and thrombin in the crystal structure of the complex (PDB ID 5DO4) [95]. An RNA-induced fit brings the PS2 moiety in close contact with the edge of Phe-232 (magenta carbon atoms) that forms a hydrophobic patch surrounded by four basic residues (side chains highlighted in ball-and-stick mode with carbon atoms colored in gray). These arginine and lysine residues generate an electric field that polarizes the thiophosphate moiety, thereby contributing to the 1000-fold tighter binding of the PS2-modified RNA to thrombin relative to the parent aptamer.