Corresponding Author

Key Words: autophagy, cardiac hypertrophy, FYCO1, heart failure
Protein homeostasis plays an essential role in maintaining normal cell function, and the disruption of protein homeostasis leads to the development of numerous diseases, including heart failure. Cells possess multiple protein quality control mechanisms, including the ubiquitin-proteasome system and the autophagy-lysosome system. While the ubiquitin-proteasome system targets soluble misfolded or aggregated proteins, large protein aggregates and organelles are degraded by the autophagy-lysosome system. Stimulation of autophagy at appropriate levels is essential for maintaining cardiac function in the presence of stress. Unfortunately, however, autophagy is activated only transiently in response to pathological stimuli and is inactivated below physiological levels thereafter, due to activation of inhibitory mechanisms or exhaustion of the autophagy core machinery. This, in turn, triggers accumulation of toxic materials, organelle dysfunction, and eventual heart failure. Disruption of protein quality control mechanisms, including insufficient autophagy, is a final common feature of heart failure caused by many different mechanisms. Although several interventions have been proposed to restore the level of autophagy in the stressed heart, current knowledge regarding the regulatory mechanism of autophagy during stress appears to be insufficient.
In this issue of the JACC: Basic to Translational Science, in order to identify cardiac enriched genes that act in a salutary manner in the presence of cardiac hypertrophy and heart failure, Kuhn et al. (1) conducted a bioinformatics search of expressed sequence tag databases. They identified several sequences corresponding to FYCO1 (FYVE and coiled-coil domain-containing protein 1), a gene highly enriched in human cardiac and skeletal muscle and known to interact with LC3, Rab7, and phosphatidylinositol-3-phosphate (PI3P), key players in autophagy. Kuhn et al. (1) focused on the functional role of FYCO1 in cardiac autophagy. They reported that FYCO1 potently stimulates autophagy in cardiomyocytes. Although systemic down-regulation of FYCO1 in mice exacerbated heart failure, cardiac-specific up-regulation of FYCO1 alleviated cardiac dysfunction in response to pressure overload (PO). The authors propose that FYCO1 performs salutary actions that alleviate cardiac dysfunction in response to cardiac stress.
FYCO1 is an adapter protein carrying the LC3-, Rab7-, and PI3P-interacting domains (2) and the coiled-coil domain that is essential for interaction with microtubules, most likely through kinesin. The unique ability of FYCO1 to interact with both autophagosomes and microtubules facilitates both autophagosome formation and transport of autophagosomes along microtubules.
Kuhn et al. (1) showed convincing evidence that FYCO1 promotes both autophagosome formation and autophagic flux in the heart during PO. Overexpression of FYCO1 increased the size and number of green fluorescent protein–fused LC3 puncta and the density of LC3 bands in immunoblot analyses. In addition, FYCO1 stimulates autophagic flux by facilitating the transport of autophagosomes. The effect of FYCO1 appears different from existing interventions to stimulate autophagy in cardiomyocytes, which act primarily on autophagosome formation. We speculate that the presence of FYCO1 promotes association of autophagosomes with microtubules, which allows cells to increase both the capacity and the flux of autophagy. PO and consequent oxidative stress markedly stimulate protein turnover. We have shown recently that autophagy is stimulated rapidly by PO but that its activity then decreases rapidly and is suppressed below baseline within a few days (3). Thus, supplementation of FYCO1, by making the autophagic process more efficient, would be extremely beneficial under such conditions.
It has been proposed that the FYCO1 protein complex competes for autophagosome and Rab7 binding with another protein (dynactin) complex containing RILP (Rab-interacting lysosomal protein) and dynein, so that autophagosomes are transported along microtubules in opposite directions depending on which protein complex dominates (2). Thus, we speculate that transport of autophagosomes to plus ends of microtubules located at the periphery of cardiomyocytes is facilitated when FYCO1 is up-regulated. It is possible that accumulation of autophagosomes in the center of the cytosol is cytotoxic. It would be interesting to investigate whether the movement of autophagosomes toward the cell periphery is observed when FYCO1 is up-regulated, and, if so, whether the localization of lysosomes is co-regulated and what their functional significance is.
Kuhn et al. (1) show that systemic Fyco1 knockout mice are normal at baseline. Whether FYCO1 is dispensable at baseline because the baseline activity of autophagy is so low in the heart or because baseline autophagy in the heart utilizes a FYCO1-independent mechanism for autophagosome formation and transport remains to be elucidated.
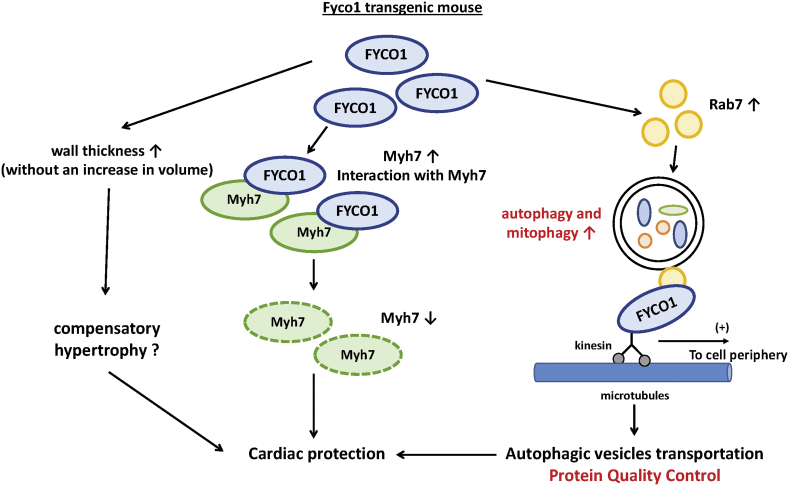
Whether the salutary effect of FYCO1 during pressure overload is predominantly mediated through stimulation of autophagic flux remains to be confirmed by conducting loss of autophagy function experiments. As Kuhn et al. noted, we speculate that other autophagy-independent actions of FYCO1 may also contribute to the salutary actions of FYCO1 during PO. For example, FYCO1 may induce compensatory hypertrophy: overexpression of FYCO1 is sufficient to induce mild cardiac hypertrophy without cardiac dysfunction at baseline; conversely, although cardiac dysfunction and chamber dilation were exacerbated in Fyco1–/– mice in response to PO, no compensatory hypertrophy was induced. Because overexpression of FYCO1 does not induce hypertrophy in cultured cardiomyocytes, it is likely that FYCO1 promotes hypertrophy non–cell autonomously, such as by stimulating paracrine mechanisms. Furthermore, the authors found that FYCO1 interacts with Myh7, thereby reducing the level of Myh7. Although the functional significance of this observation remains to be clarified, it raises the possibility that FYCO1 has multiple functions through protein-protein interactions (Figure 1).
Figure 1.
Potential Molecular Mechanisms Through Which FYCO1 May Mediate Protective Effects in the Mouse Heart During Pressure Overload
The salutary effects of FYCO1 (FYVE and coiled-coil domain-containing protein 1) overexpression in the heart during pressure overload may be mediated through multiple mechanisms, including stimulation of autophagy, induction of compensatory hypertrophy, and interaction with Myh7. FYCO1 appears to stimulate autophagosome formation and promote microtubule-dependent transport of autophagosomes. Although the cardioprotective effect of FYCO1 during pressure overload is mediated primarily through stimulation of autophagy, other mechanisms may also be involved.
Microtubules are dynamically formed by polymerization of alpha- and beta-tubulin dimers and regulate various cellular processes, including cell shape, mitosis, and protein transport. The density of microtubules is generally increased, and alpha-tubulin is acetylated in the presence of PO or cardiac hypertrophy. Although accumulation of stable microtubules contributes to increased mechanical resilience through stabilization of myofibril structure and better protein quality control through the enhancement of autophagy, it is well known that microtubule polymerization negatively affects cardiac contraction (4). We speculate that FYCO1 plays an essential role in promoting the salutary action of microtubules during PO. Interestingly, class I histone deacetylase inhibitors, which attenuate cardiac dysfunction during mechanical overload, have the ability to promote autophagosome transport along microtubules (5). Thus, protein quality control mechanisms mediated through microtubules, including autophagosome transport, are promising targets for heart failure treatment, and further investigation is warranted.
The authors report that the level of FYCO1 is increased in the mouse model of dilated cardiomyopathy, whereas FYCO1 was down-regulated in human cardiomyopathy samples. We speculate that FYCO1 is up-regulated during the acute phase of stress, but this needs to be confirmed experimentally. Considering the salutary actions of FYCO1 in the heart, it would be interesting to elucidate the mechanism through which the level of endogenous FYCO1 in the heart is regulated in response to stress. This may lead to the development of an intervention to maintain the level of endogenous FYCO1 under end-stage heart failure conditions.
In summary, FYCO1 is necessary and sufficient to protect the heart against PO by promoting autophagy and mitophagy. Overexpression of FYCO1 is a novel and effective way of enhancing autophagic flux in the heart and, thus, has a strong therapeutic potential. It would be interesting to test whether an intervention to up-regulate FYCO1 or FYCO1 itself is beneficial in other cardiac conditions, such as post–myocardial infarction cardiac remodeling. Furthermore, given its interaction with microtubules and other cytoskeletal proteins and its effect on compensatory hypertrophy, FYCO1 may also work through additional mechanisms to protect the heart against hemodynamic overload. Further investigations are warranted to clarify additional functions of FYCO1 in the heart.
Funding Support and Author Disclosures
This work was supported in part by U.S. Public Health Service Grants HL67724, HL91469, HL102738, HL112330, HL138720, HL144626, HL150881, and AG23039 (to Dr. Sadoshima); American Heart Association Merit Award, 20 Merit 35120374 (to Dr. Sadoshima); and the Fondation Leducq Transatlantic Network of Excellence 15CBD04 (to Dr. Sadoshima). Dr. Mukai has been supported by a Postdoctoral Fellowship from the American Heart Association (18POST34060247). Both authors have reported that they have no relationships relevant to the content of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Kuhn C., Menke M., Senger F. FYCO1 regulates cardiomyocyte autophagy and prevents heart failure due to pressure overload in vivo. J Am Coll Cardiol Basic Trans Science. 2021;6:365–380. doi: 10.1016/j.jacbts.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pankiv S., Alemu E.A., Brech A. FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end-directed vesicle transport. J Cell Biol. 2010;188:253–269. doi: 10.1083/jcb.200907015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shirakabe A., Zhai P., Ikeda Y. Drp1-dependent mitochondrial autophagy plays a protective role against pressure overload-induced mitochondrial dysfunction and heart failure. Circulation. 2016;133:1249–1263. doi: 10.1161/CIRCULATIONAHA.115.020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsutsui H., Ishihara K., Cooper G., 4th Cytoskeletal role in the contractile dysfunction of hypertrophied myocardium. Science. 1993;260:682–687. doi: 10.1126/science.8097594. [DOI] [PubMed] [Google Scholar]
- 5.Blakeslee W.W., Lin Y.H., Stratton M.S. Class I HDACs control a JIP1-dependent pathway for kinesin-microtubule binding in cardiomyocytes. J Mol Cell Cardiol. 2017;112:74–82. doi: 10.1016/j.yjmcc.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]