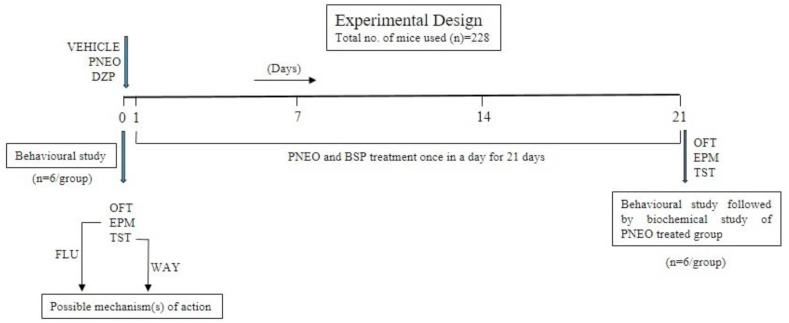
Figure 1.
Schematic illustration of the animal experimental design. In the acute study, vehicle (control) and Piper nigrum EO (PNEO 5, 10 and 50 mg/kg) were orally administered. The reference drug, Diazepam (DZP 1 mg/kg) was administered intraperitoneally in the behavioural studies like Open field test (OFT), Elevated plus maze (EPM) and Tail suspension test (TST). To investigate the possible mechanism of action the antagonist flumazenil (FLU 3 mg/kg) and WAY-100635 (WAY 1 mg/kg) were administered intraperitoneally 15 min before oral treatment. In repetitive study, Control, PNEO (5, 10 and 50 mg/kg, p.o) and reference drug Buspirone (BSP 10 mg/kg, i.p) were administered once in a day for 21 days. On the last day, behavioural and biochemical study were performed.