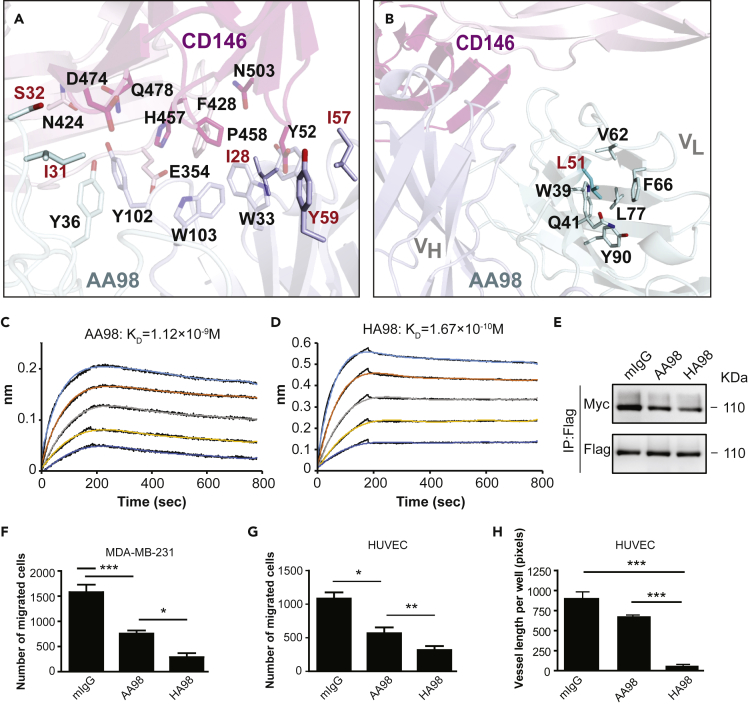
Figure 4.
Rational optimization of AA98 based on the interface between AA98 Fab and CD146 D4-D5
(A) Interface between CD146 D4-D5 and AA98 Fab. AA98 Fab and CD146 D4-D5 are shown in cartoon representation, and residues involved in interaction are shown in stick representation. CD146 D4 is colored in light pink, whereas D5 is colored in hot pink. The Fab light chain and heavy chain are colored in pale cyan and light blue, respectively. Residues in AA98 involved in mutation were labeled in red.
(B) AA98 residues around the L51 (shown in sticks) in light chain, which are distal to the CD146 and AA98 interface.
(C and D) BLI-binding assay was used to detect the affinity of wild-type AA98 (C) and modified HA98 (D).
(E) The effect of HA98 on dimerization of CD146 analyzed by Western blot with double-tag system. HUVECs transfected with full-length CD146 with either an FLAG tag or a Myc tag were treated with AA98 or HA98 and then subjected to the double-tag system immunoprecipitation and Western blotting assay.
(F and G) MDA-MB-231 (F) and HUVECs (G) treated with AA98 or HA98 were subjected to a transwell migration assay. Data were represented as mean ± standard deviation. Significant differences were determined by one-way analysis of variance (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ns, no significant difference). Representative images are shown in Figure S9.
(H) HUVECs treated with AA98 or HA98 were subjected to the tube formation assay. Data were represented as mean ± standard deviation. Significant differences were determined by one-way analysis of variance (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ns, no significant difference).
See also Figures S8 and S9.