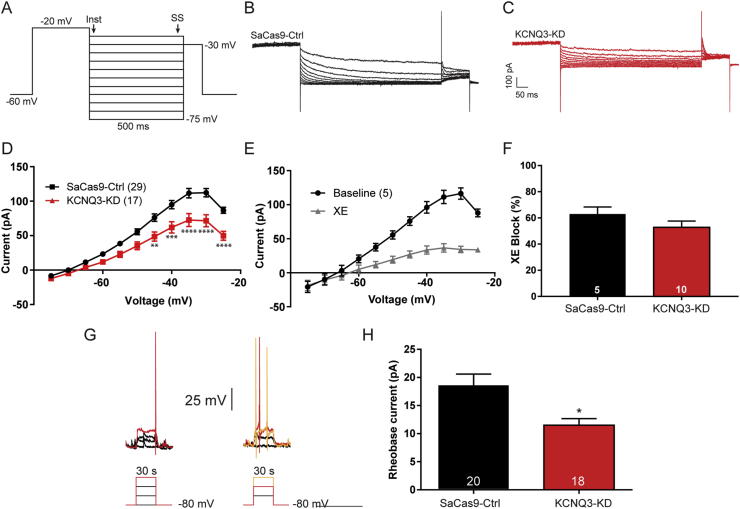
Figure 3.
CRISPR gene editing of Kcnq3 decreased the M-current in OVX EB-treated female mice. (A) The deactivation protocol in voltage clamps started by holding the cell at −60 mV before a voltage step to −20 mV (300 ms), followed by 5 mV incremental steps (−25 to −75 mV, 500 ms). The current measured during the steady state (ss) was subtracted from the instantaneous (Inst) current. TTX (1 μM) was present in the bath. (B and C) The sag seen following the voltage step was the deactivation of the M-current, which was higher in the control animals (B) compared to the KCNQ3-KD animals (C). (D) Graphed as an I–V plot, the M-current was smaller across a range of voltages in the KCNQ3-KD mice (two-way ANOVA, main effect of group, F(1, 484) = 93.40) with significant post hoc differences between −45 and −25 mV (Bonferroni's test). (E) The M-current was strongly inhibited after 10 min of bath application of 40 μM XE 991, a selective blocker of KCNQ channels. (F) The area under the curve for the deactivation protocol was calculated before and after XE application to determine the percent block of the current. The XE sensitive current was the M-current with the residual current due to other K+ conductance. There was no difference in the effectiveness of XE between the control (62.9 ± 5.5%) and KCNQ3-KD (52.8 ± 4.9%) cells (unpaired t-test, t(13) = 1.360). Error bars indicate SEM. (G) In current clamps, the rheobase was assessed in both Cas9-control and Cas9-KCNQ3. The membrane potential was held at −80 mV before small amounts of current were injected (∼6 pA/step, 500 ms). The red current trace represents the minimum current necessary to elicit an action potential. (H) The amount of current required to elicit a spike was significantly greater in the controls than KCNQ3-KD cells (18.4 ± 2.2 vs 11.5 ± 1.2 pA, n = 20/18) even when normalized for capacitance (1.3 ± 0.1 vs 0.9 ± 0.1 pA/pS). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001. Number of cells per group is indicated in each panel. Error bars indicate SEM.