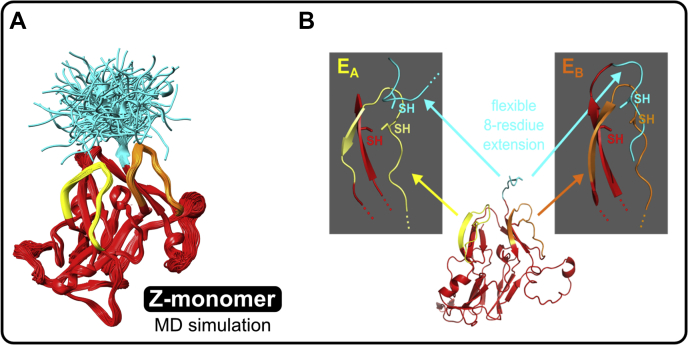
Figure 9.
Molecular dynamic (MD) simulations predict the Z-appendage can assume multiple conformations.A, a molecular dynamics simulation (1000 independent runs) (cyan) was performed to sample conformations of the Zurich variant. B, MD simulation analysis revealed two clusters of conformants of the 8-residue extension particularly close to the EA or EB regions. This confirms that free cysteine residue within the extension can form alternative disulfide bridges and disturb other interactions within the monomer. Interference with disulfide formation can lead to conformational changes of the EA and EB regions leading to appearance of immunogenic neoepitopes.