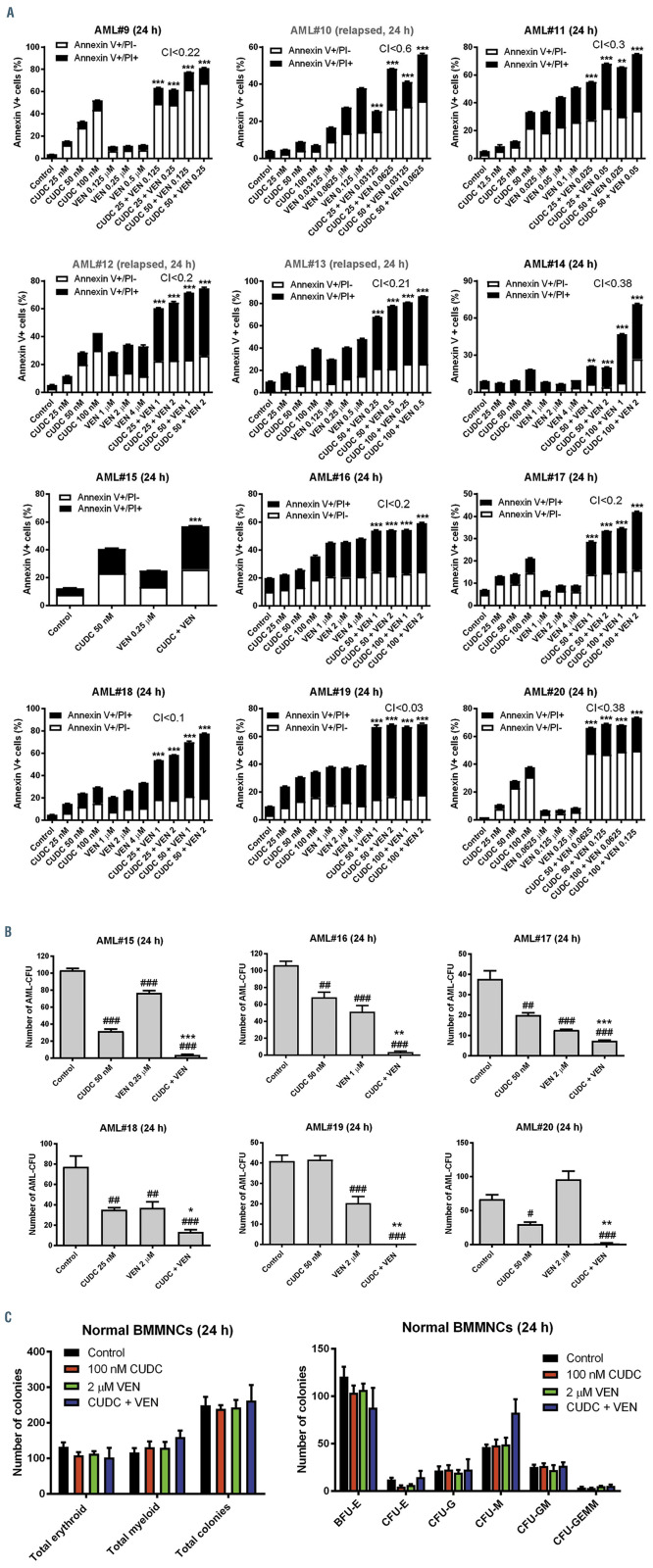
Figure 2.
CUDC-907 and venetoclax synergize in primary acute myeloid leukemia cells and cooperatively prevent colony formation of acute myeloid leukemia progenitor cells while sparing normal hematopoietic progenitor cells ex vivo. (A) Primary acute myeloid leukemia (AML) patient samples were treated with vehicle control, venetoclax (VEN), CUDC-907 (CUDC), or in combination for 24 hours. Flow cytometry analysis of Annexin-V-FITC/PI staining was performed. Results are shown as mean percent Annexin V+ cells ± standard error of the mean (SEM). Combination index (CI) values were calculated using CompuSyn software. **P<0.01 and ***P<0.001 compared to single drug treatments. (B) Primary AML patient samples were cultured with vehicle control, venetoclax, CUDC-907, or in combination for 24 hours and then plated in methylcellulose. After incubation for 10-14 days, the number of surviving AML cells capable of generating leukemia colonies (AML-CFU) were enumerated. Data are presented as mean ± SEM. #P<0.05, ##P<0.01, and ###P<001 compared to control. *P<0.05, *P<0.01, and ***P<0.001 compared to single drug treatments. Technical triplicates were performed. (C) Normal human bone marrow mononuclear cells from a single donor were cultured with vehicle control, venetoclax, CUDC-907, or in combination for 24 hours, and then plated in methylcellulose. After incubation for 10-14 days, the number of surviving hematopoietic cells capable of generating colonies were enumerated. Total erythroid and myeloid colonies are presented as mean ± SEM (left panel). The number of BFU-E, CFU-E, CFU-G, CFU-M, CFU-GM, and CFU-GEMM colonies are presented as mean ± SEM (right panel).