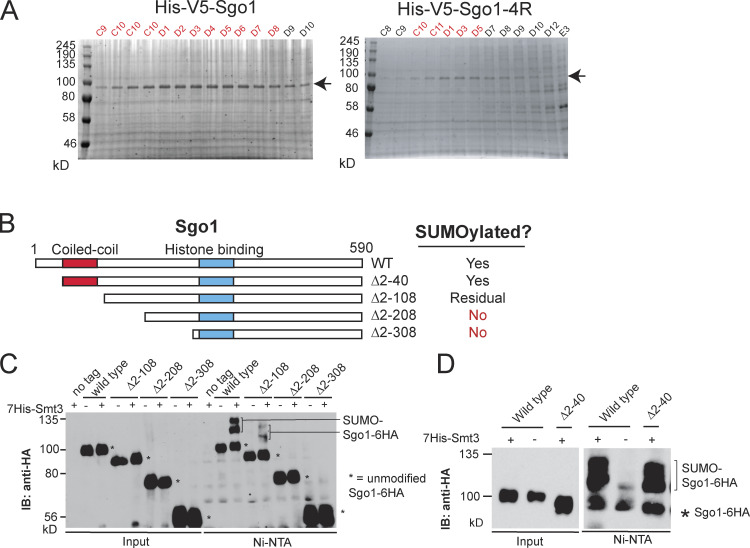
Figure S2.
Recombinant Sgo1 was successfully purified and maximum Sgo1 SUMOylation required the coiled-coil domain. (A) Purification of Sgo1. Coomassie staining confirmed the successful purification of wild-type and Sgo1-4R proteins. (B) Schematics describing the truncation mutants generated for Sgo1. The conserved coiled-coil and basic domains are highlighted in red and blue, respectively. Results from C and D are summarized on the right. (C) The first 208 amino acids are essential for Sgo1 SUMOylation. In vivo SUMOylation was assessed for the following Sgo1-6HA tagged strains as described in Fig. 2 A, together with the indicated negative controls: wild type (AMy7654), sgo1Δ2-108 (AMy14764), sgo1Δ 2–208 (AMy14765), and sgo1Δ2-308 (AMy14766). Unmodified Sgo1 bands are marked with asterisks. IB, immunoblot. (D) The coiled-coil domain is required for maximum Sgo1 SUMOylation. In vivo SUMOylation was assessed for the following Sgo1-6HA tagged strains: wild type (AMy7654) and sgo1Δ2-40 (AMy18194).