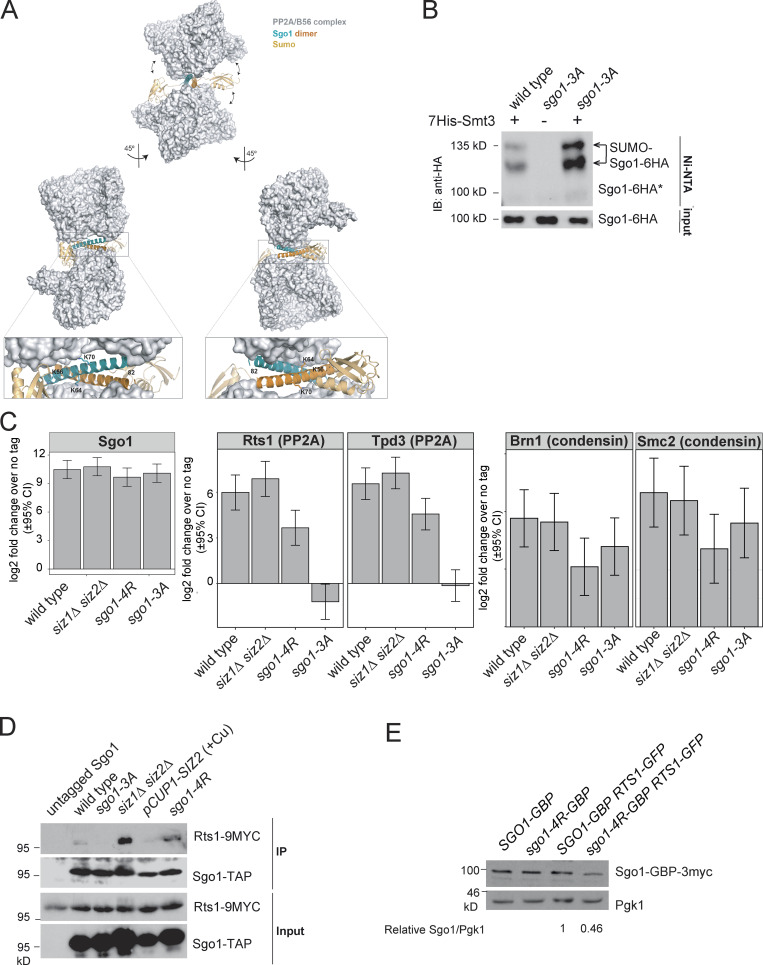
Figure S3.
SUMOylation disrupts Sgo1–Rts1 interaction. (A) Structural modeling predicts that SUMOylation on the coiled-coil domain of Sgo1 is incompatible with Sgo1–PP2A interaction. S. cerevisiae Sgo1–PP2A interaction was modeled based on structural information obtained from cocrystallized human Sgo1(51–96) and PP2A using Phyre2 web portal (Kelley et al., 2015). Potential consequences of SUMOylation were modeled using the molecular graphic program PyMOL (v2.0; Schrödinger). According to this model, Lys64 and Lys70 are critically positioned at the binding surface with no room to accommodate a bulkier modification such as SUMOylation. Lys56 is exposed to the solvent, but the attachment of SUMO (highlighted in gold) is expected to result in steric clashes with PP2A and weaken Sgo1-PP2A binding. Structural information is unavailable beyond Leu82, so Lys85 could not be included in this model. (B) Rts1 binding is not required for Sgo1 SUMOylation. 6-HA–tagged wild type (AMy906) and Sgo1-3A(AMy25988) were analyzed as described in Fig. 2 A. IB, immunoblot. (C) Global Sgo1–Rts1 interaction was mildly increased in siz1Δ siz2Δ and moderately reduced in sgo1-4R. Global Sgo1–Brn1 interaction was intact in siz1Δ siz2Δ and moderately reduced in sgo1-4R. Strains used carried 6His-3Flag–tagged Sgo1: wild type (AMy23137), siz1Δ siz2Δ (AMy29146), sgo1-4R (AMy26329), and sgo1-3A (AMy29203). Cells were arrested in benomyl at 30°C, and proteins copurifying with Sgo1-6His-3Flag were analyzed by label-free quantitative MS in triplicate. Data were analyzed by the DEP R package, and error bars represent standard deviation. CI, confidence interval. (D) Global Sgo1–Rts1 interaction was increased in siz1Δ siz2Δ and reduced in the SIZ2-overexpressing strain. sgo1-4R did not show a strong impact on the interaction. Cycling cells grown at room temperature were used for co-IP analysis as described in Materials and methods. Strains used carried Sgo1-TAP and Rts1-9Myc and were wild type (AMy9144), sgo1-3A (AMy9145), siz1Δ siz2Δ (AMy21943), pCUP1-SIZ2 (AMy27970), and sgo1-4R (AMy26090) or untagged Sgo1 (AMy4721). Sgo1-TAP and Rts1-9Myc were detected by PAP and anti-Myc Western blotting, respectively. (E) Sgo1-4R expression level was reduced when Rts1-GBP was also present. Protein extracts from strains used for Fig. 9, E–G, were analyzed by Western blot. Signal intensities were measured using ImageJ. Ratios of Sgo1-GBP-3myc to Pgk1 were calculated, and the intensity for SGO1-GBP-3MYC RTS1-nfGFP was set to 1.