Figure 1.
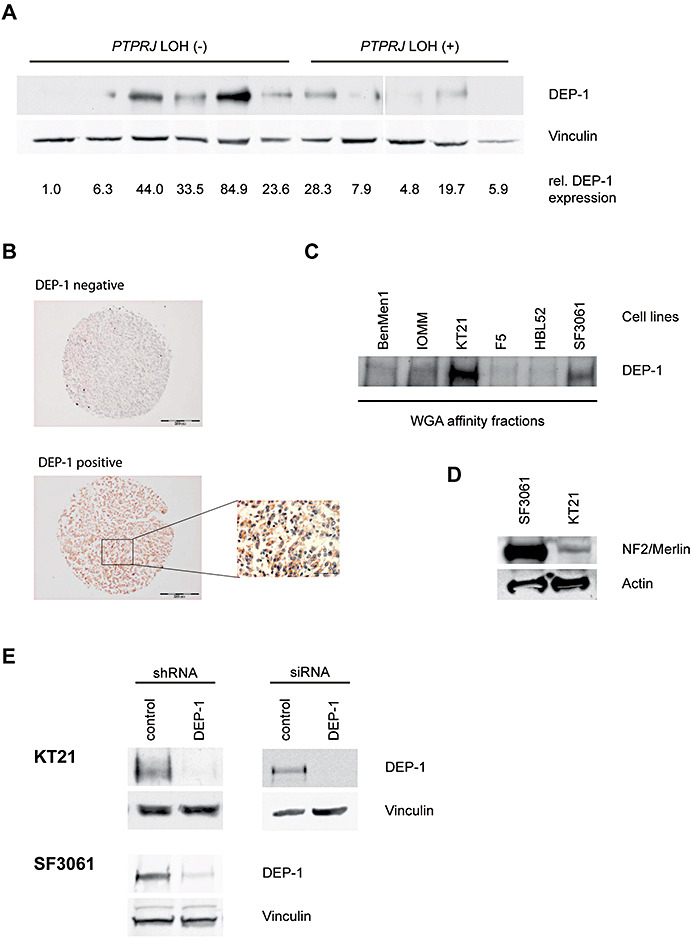
A. Lysates of meningioma tumor samples with or without LOH at the PTPRJ locus, as indicated, were analyzed for DEP‐1 expression. Equal amounts of lysate protein were subjected to enrichment of DEP‐1 by wheat‐germ agglutinin affinity precipitation and analyzed by immunoblotting using anti‐DEP‐1 143‐41 monoclonal antibodies. DEP‐1 expression was quantified by densitometry and normalized to the intensity of the loading control vinculin. All lanes were on the same blot with identical exposure and image processing, but were rearranged for better clarity. B. Immunostainings of paraffin‐embedded meningioma samples derived from a tissue microarray. One example for positive and negative staining each is shown (scale bars 200 µm), the inset is a larger magnification (scale bar 50 µm). C. DEP‐1 protein expression in meningioma cell lines (details on the cell lines given in the Materials and Methods section) was analyzed by immunoblotting. Equal protein amounts of the meningioma cell lysates were subjected to enrichment by wheat‐germ agglutinin affinity precipitation. D. Cell lysates from SF3061 and KT21 cells were tested for NF2/Merlin expression by immunoblotting. E. Stable knockdown of DEP‐1 in KT21 and SF3061 cells was achieved by lentiviral transduction with DEP‐1 shRNA (or non‐targeting control shRNA) expression constructs and subsequent puromycin selection of transduced cells. For transient knockdown of DEP‐1 in the KT21 cell line, cells were transfected with a DEP‐1 siRNA (or a non‐targeting control siRNA). Equal protein amounts of the cell lysates were subjected to enrichment by wheat‐germ agglutinin affinity precipitation and analyzed by immunoblotting.
