Figure 8.
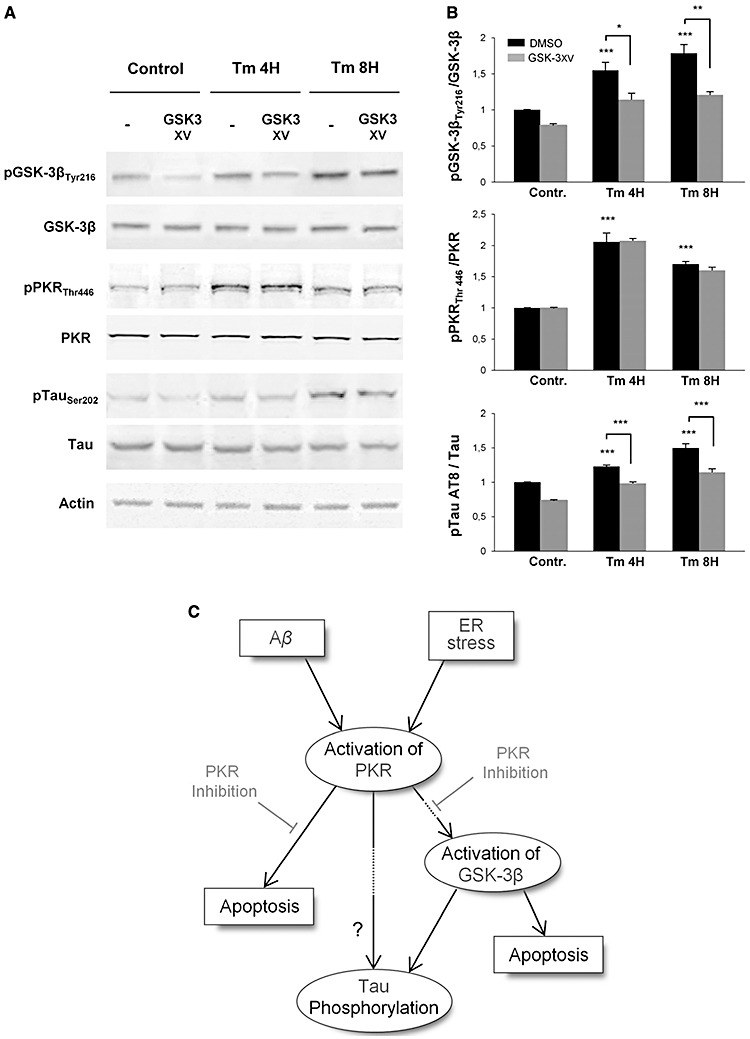
Western‐blot analysis of pGSK‐3 inhibitor (GSK‐3 XV) effect after Tm exposure. Protein extracts from SH‐SY5Y cells were incubated with anti‐GSK‐3β, anti‐pGSK‐3βTyr216, anti‐pPKRThr446, anti‐PKR, anti‐pTau (AT8), anti‐Tau and anti‐actin primary antibodies. A. GSK‐3 XV treatment on SH‐SY5Y cells inhibit GSK‐3β phosphorylation but does not affects the phosphorylation levels of PKR. However, pretreatment with this inhibitor markedly attenuates Tau phosphorylation on AT8 site. B. The blots were quantified using Multigauge software, and results obtained were normalized on actin levels. pGSK‐3βTyr216/GSK‐3β ratio showed that GSK‐3 XV inhibitor decreases by around 32% the phosphorylation level of GSK‐3β, without any modification on PKR activation. Quantified levels of phosphorylation of Tau on AT8 site confirmed that pTau gradually increases with ER stress induces by Tm, and revealed that this activation is significantly inhibited at each time point by the GSK‐3 XV. The experiments were performed in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001. C. Schematic diagram of the potential signaling pathways activated by Tm and Aβ linked to PKR, GSK‐3β and tau in SH‐SY5Y cells. Only PKR has been represented but other stress‐associated kinases (such as JNK, P38 and ERK not presented here) could also participate in tau phosphorylation and /or apoptosis.
