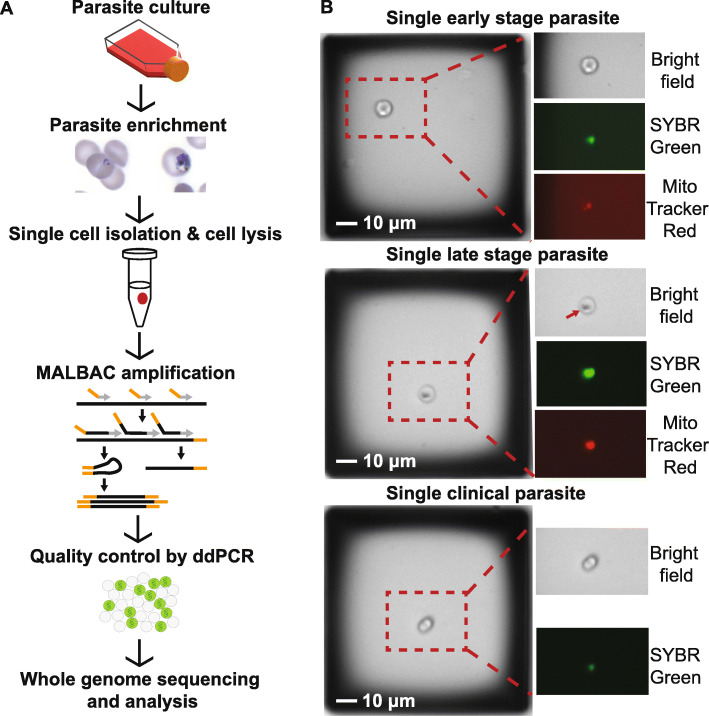
Fig. 1.
Single P. falciparum-infected erythrocytes are isolated, amplified, and sequenced. a Experimental workflow. Parasites are grown in vitro in human erythrocytes or isolated from infected patients. To limit the number of uninfected erythrocytes in the sample, infected cells are enriched using column and gradient-based methods (see “Methods”). Individual early-stage (left image) and late-stage (right image) parasite-infected erythrocytes were automatically isolated into PCR tubes using the CellRaft AIR System (Cell Microsystems, see panel b). All cells were lysed and amplified by MALBAC. MALBAC uses a combination of common (orange) and degenerate (grey) primers to amplify the genome. The quality of amplified genomes was assessed prior to library preparation and sequencing using droplet digital PCR (ddPCR); DNA is partitioned into individual droplets to measure gene copies. Samples were Illumina sequenced and analyzed as detailed in Additional file 1: Figure S2. b Parasite stage visualization on the CellRaft AIR System using microscopy (× 10 magnification). Enriched early- and late-stage parasite-infected erythrocytes at low density were seeded into microwells to yield only a single cell per well (left image of each group), and identified with SYBR green and Mitotracker Red staining (indicates parasite DNA and mitochondrion, respectively). Early-stage parasites exhibited lower fluorescence due to their smaller size, and late-stage parasites had noticeable dark spots (arrow) due to the accumulation of hemozoin pigment. Scale bar represents 10 μm.