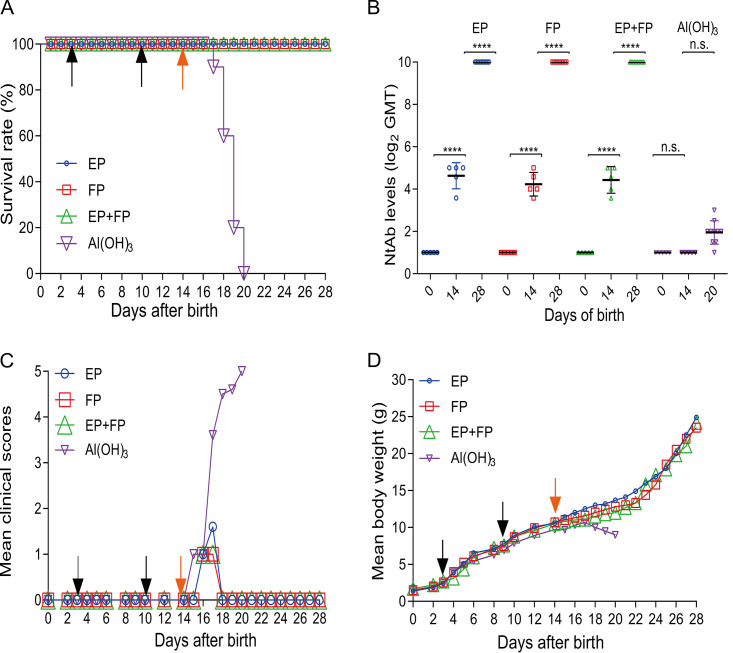
FIG 4.
Efficacy of the vaccine in mice immunized with the three vaccine formulations. Kunming mice were primed and boosted on days 3 and 10 at a dose of 1.5 μg/injection and challenged at a dose of 10 LD50 (2 × 106 CCID50/mouse) on day 14. (A) The survival rate (percent) was calculated 14 days postchallenge. Bleeding was performed on days 0, 14, and 28. NtAb titers of sera of mice vaccinated with EPs, FPs, EPs+FPs, or Al(OH)3 (n = 5 for each group) were determined. NtAb titers on day 28 in mice infected with EPs, FPs, or EPs+FPs (n = 10 for each group) were determined. NtAb titers are presented as the means ± SEM. ****, P < 0.0001; n.s., not significant (by two-way ANOVA). (B) NtAb titers below 8 were assigned to 2 for the convenience of presentation. Mean clinical scores (C) and mean body weight (D) were recorded during the 14-day observation period. Black arrows indicate the date of vaccination. Orange arrows indicate the day of challenge.