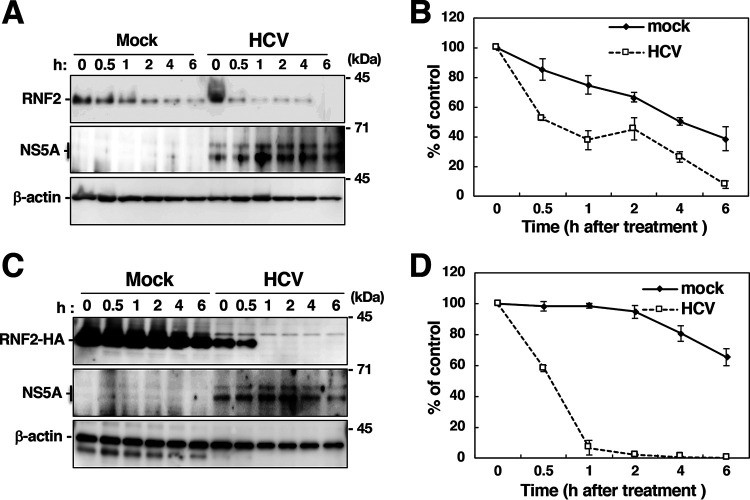
FIG 5.
Cycloheximide chase assay to evaluate the stability of the RNF2 protein. (A) Mock-infected and HCV-infected cells were incubated in medium containing 10 μg/ml cycloheximide (CHX) and were then harvested at each indicated time. Cell lysates were prepared from the harvested cells and were then subjected to Western blotting. (B) The intensities of the RNF2 bands shown in panel A were quantified using ImageJ software and standardized with individual beta-actin intensity. The data shown are representative of three independent experiments and are presented as the mean ± SD values (n = 3). (C) A plasmid encoding RNF2-HA was transfected into mock-infected cells and HCV-infected cells. CHX was added to the medium at 36 h posttransfection. The resulting cells were harvested at the indicated times to prepare cell lysates, which were subjected to Western blotting. (D) The intensities of the RNF2-HA bands in each group shown in panel C were quantified using software and standardized with individual beta-actin intensity. The data shown are representative of three independent experiments and are presented as the mean ± SD values (n = 3).