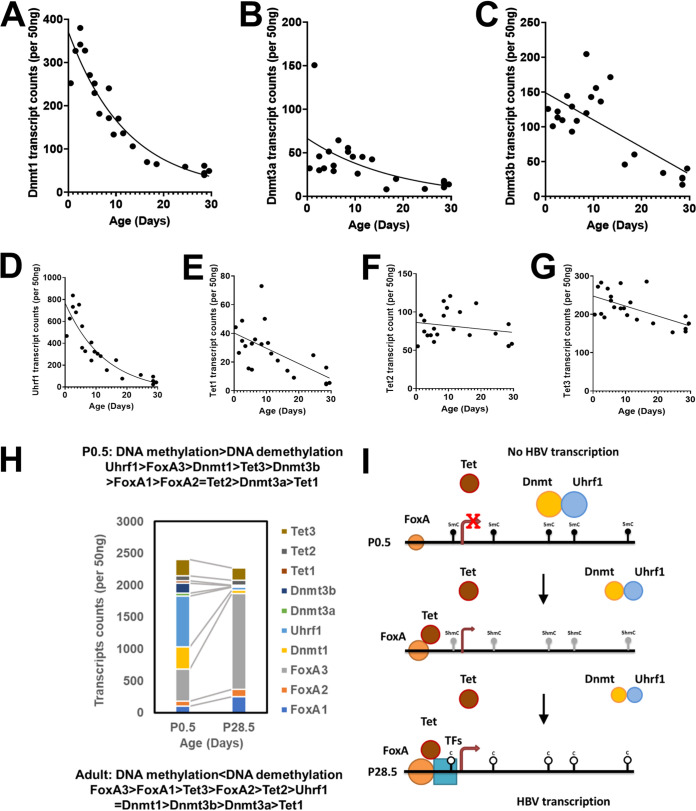
FIG 7.
Developmental expression of DNA methyltransferase and ten-eleven translocation methylcytosine dioxygenase transcript levels in livers of HBV transgenic mice. Quantitative analysis of the liver Dnmt1 (A), Dnmt3a (B), Dnmt3b (C), Uhrf1 (D), Tet1 (E), Tet2 (F), and Tet3 (G) transcripts by NanoString gene expression analysis in the HBV transgenic mice. One-phase decay analysis was used to estimate the changes in gene expression throughout postnatal liver development in the HBV transgenic mice (GraphPad Prism 8.4). (H) Stacked column chart indicating the relative abundances of FoxA1, FoxA2, FoxA3, Dnmt1, Dnmt3a, Dnmt3b, Uhrf1, Tet1, Tet2, and Tet3 in neonatal (postnatal day 0.5) and adult (postnatal day 28.5) HBV transgenic mice (Fig. 1 and 7). (I) Model for the developmental regulation of HBV DNA methylation and transcription. In the neonatal (P0.5) wild-type HBV transgenic mice, the HBVtransgene DNA is maintained in an extensively methylated state by Dnmt1 and Uhrf1 with possible additional contributions from Dnmt3a and Dnmt3b. FoxA is expressed at birth and marks the HBV genome for later developmental expression upon recruitment of additional epigenetic-modifying activities and transcription factors to the viral promoters. During postnatal development, FoxA expression levels increase modestly while Dnmt levels rapidly decline, permitting the recruitment of additional transcriptional factors to the viral enhancer and promoter sequences. FoxA alone, or in combination with the additional transcription factors, leads to Tet recruitment and the oxidative conversion of 5mC to 5hmC. The conversion of 5mC to 5hmC, in combination with declining Dnmt activity, in the presence of hepatocyte proliferation leads to both active and passive demethylation of HBV DNA throughout postnatal liver development. In adult HBV transgenic mice, viral DNA is maintained in an unmethylation state due to the balance between Tet-mediated demethylation and Dnmt-dependent methylation.