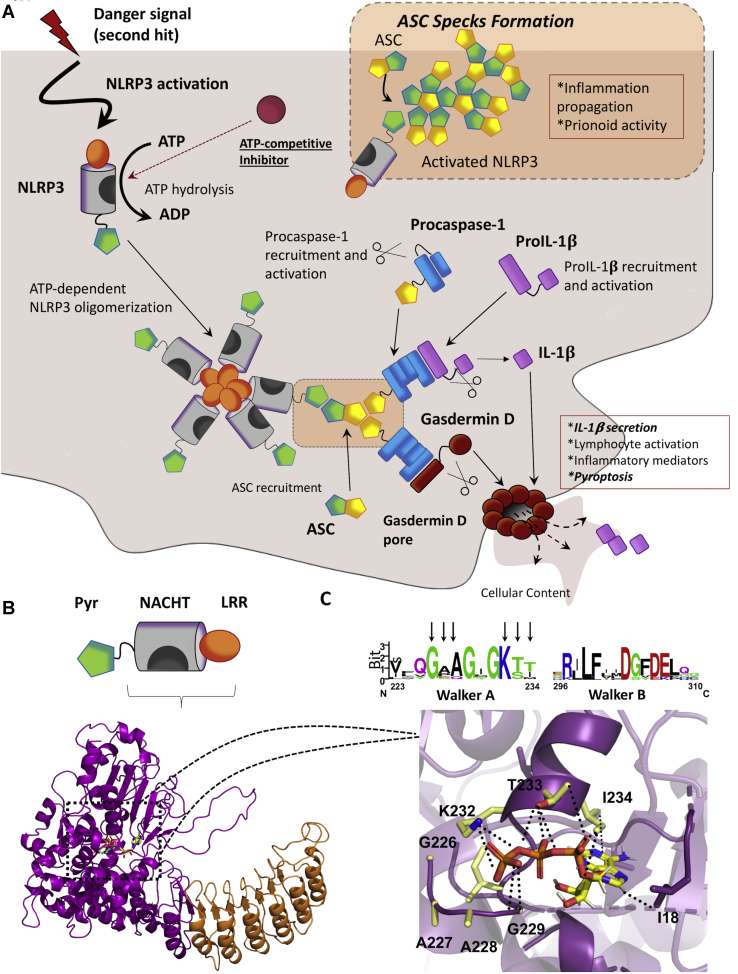
Figure 1.
The NLRP3 inflammasome.A, the NLRP3 inflammasome activation pathway. Generally, the NLRP3 inflammasome might be activated by a two-signal mechanism; the first signal (not shown in this scheme) increases the expression of the different components of the inflammasome. The second signal might trigger the activation and the assembly of the inflammasome. First, ATP binds to the nucleotide-binding and oligomerization (NACHT) domain, being subsequently hydrolyzed. Activated NLRP3 oligomerizes and recruits the apoptosis-associated speck-like protein containing a CARD (ASC). ASC is a scaffolding protein and recruits procaspase-1, allowing its autoproteolytic activation. Finally, activated caspase-1 cleaves pro-IL-1β into IL-1β, which is secreted and exerts inflammatory effects. Active caspase-1 also recruits and induces the activation of Gasdermin-D, which forms pores in the cell membrane, leading to the release of the cellular content and, subsequently, pyroptosis. In addition, activated ASC nucleates into large specks that have prionoid properties and contribute to inflammation propagation. Processes representing readouts for inflammasome activation are showed in italics. B, NLRP3 is organized in three different domains; a C-terminal leucine-rich repeat (LRR), a central NACHT, and N-terminal pyrin domain (PYD). The 3D model for NLRP3 is shown in the cartoon, with the bound ATP as green sticks. The model was built by homology modeling, using as a template the crystal structure of the nucleotide-binding oligomerization domain-containing protein 2 (NOD2). C, top, the sequence logo for the multiple sequence alignment of the highly conserved Walker A and Walker B motifs. NLRP3 numeration is used. Bottom, detail of the nucleotide-binding cavity. Residues from Walker A motive close to ATP (shown as sticks) are highlighted in sticks and colored in CPK code with carbons in yellow. Electrostatic interactions between K232 and ATP phosphate groups are displayed as black dots. NLRP3, nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain–containing protein 3.