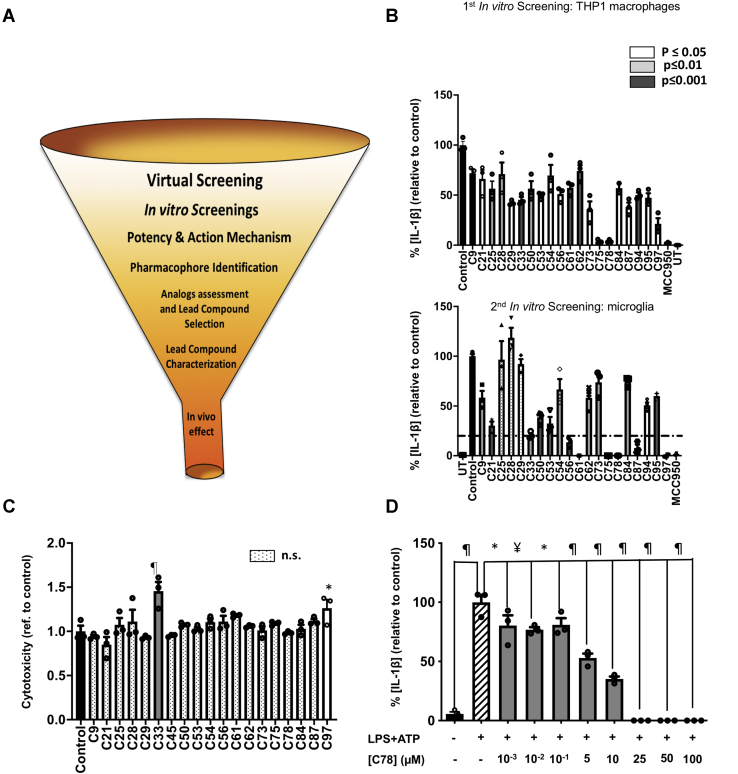
Figure 2.
In vitro screening.A, the diagram for the drug development process. The funnel represents the reduction in the number of compounds, starting from 17 million molecules in the virtual screening and finishing with a single compound to be tested in vivo. B, in vitro screenings for the 100 compounds from the in silico screening. The first screening tested the ability of the 100 molecules to inhibit NLRP3-mediated production of IL-1β by THP1 macrophages, primed, and activated with LPS and ATP, respectively. The compounds that inhibit IL-1β production with any level of statistical significance were tested in a secondary screening using murine primary microglia, primed, and activated with LPS (400 ng/ml, 3 h) and ATP (4.5 mM, 45 min). The compounds that inhibit IL-1β release below a 20% threshold were selected for further characterization. C, cytotoxicity exerted by compounds originating from the first screening, as measured by the lactate dehydrogenase released in cell supernatants, after treatment with 50 μM of the compounds. D, the dose-dependent inhibitory effect of one of the three compounds (C78), chosen according to potency criteria, to be deeper characterized. The data shown correspond to average values of biological triplicates (n = 3), ran in technical duplicates ± SEM. Statistical significances are referred to control samples; LPS + ATP in panels B and D and untreated cells in panel C. Samples and controls contained the same DMSO%. Positive controls for NLRP3 inhibition with the NLRP3 inhibitor MCC950 are included in panel B. Significance levels, as calculated by one-way ANOVA, are indicated as white (p ≤ 0.05), light gray (p ≤ 0.01), and dark gray (p ≤ 0.001) bars in panels B and C and as ∗p ≤ 0.05, ¥p ≤ 0.01, and ¶p ≤ 0.0001 in panel D. IL-1β, interleukin 1β; NLRP3, nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain–containing protein 3.