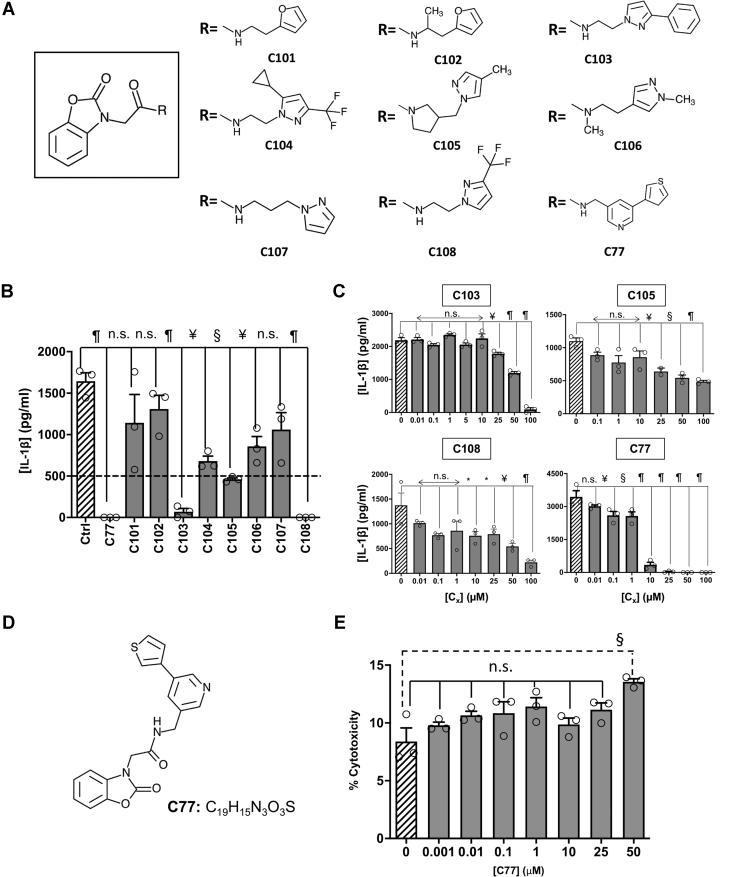
Figure 4.
Benzoxazolone acetamide analogs searching and assessment.A, the structure of the common core identified as the pharmacophore (squared). Chemical structure for the substituents of the selected benzoxazolone acetamide analogs. B, the inhibitory effect of 100 μM of each analog in NLRP3-mediated IL-1β production by primary murine microglia activated with LPS (400 ng/ml, 3 h) and ATP (4.5 mM, 45 min). The dashed line indicates 30% of activity regarding to controls. C, inhibition of NLRP3 activation by different concentrations of the selected analogs, as determined by measuring the IL-1β produced by microglia cultures. D, the structure and molecular formula of C77. E, cytotoxicity exerted by different concentrations of C77 in microglial murine cultures. The data shown correspond to average values of biological triplicates (n = 3), ran in technical duplicates ± SEM. Statistical significances are referred to control samples; LPS + ATP in panels B, C, and D and untreated cells in E. Samples and controls contained the same DMSO%. Significance levels, as calculated by one-way ANOVA, are indicated as ∗p ≤ 0.05, ¥p ≤ 0.01, §p ≤ 0.001, and ¶p ≤ 0.0001. IL-1β, interleukin 1β; NLRP3, nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain–containing protein 3.