Figure 9.
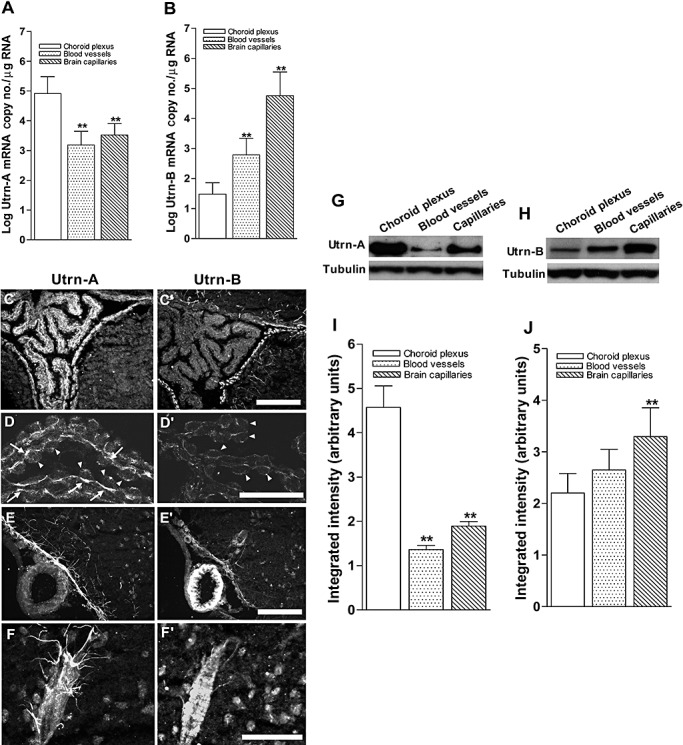
Differential expression of Utrn‐A and ‐B in choroid plexus and vascular elements. A. Expression pattern of Utrn‐A transcript was quantified in microdissected choroid plexus, central nervous system (CNS) blood vessels and fractionated capillaries. Utrn‐A transcript expression was significantly higher in choroid plexus than in blood vessels and capillaries (n = 6; **P < 0.001). Compared with blood vessels, transcript level was significantly higher in capillaries (n = 6; *P < 0.01). B. CNS capillaries showed significantly higher Utrn‐B mRNA (n = 6; **P < 0.001) than choroid plexus and blood vessels. Mean ± standard deviation (SD) are shown. C,C'. Immunolabeling of transverse section of mdx brain at the level of choroid plexus showed strong Utrn‐A immunolabeling; however, adjacent section labeled with Utrn‐B shows weak labeling in the choroid plexus. The ependymal lining and vascular elements show strong immunolabeling with both antibodies. D,D'. Higher magnification of the choroid plexus from dystrophic mouse immunolabeled with anti‐Utrn‐A (D) and anti‐Utrn‐B antibodies (D'). Strong immunolabeling of Utrn‐A along the basal membrane (arrows) and weak staining at the epithelial cells (arrow heads). Choroid plexus show weak Utrn‐B immunolabeling, which may contribute to the high vascular contents, whereas choroid epithelial cells (arrowheads) did not show Utrn‐B immunolabeling in dystrophic mice. E,E'. A blood vessel along ventral region of brain demonstrates no significant Utrn‐A labeling, whereas ependymal lining, perivascular astrocytes and neurons show moderate Utrn‐A labeling. Adjacent section reacted with Utrn‐B antibodies, showing strong immunolabeling in the blood vessels. Also note moderate staining along the ependymal lining and vascular elements. F,F'. Higher magnification of a blood vessel show Utrn‐A labeling of perivascular astrocytes making close contact with blood vessels; however, adjacent section reacted with Utrn‐B label the blood vessels rather than perivascular astrocytes. Note that moderate labeling of neurons is also visualized. G,H. Western blot analysis of lysates containing 50 µg of total protein probed with Utrn‐A and ‐B antibodies. G. Consistent with immunolabeling, Utrn‐A is expressed at high levels in choroid plexus compared with capillaries and blood vessels. H. Differential expression of Utrn‐B protein in choroid plexus, blood vessels and capillaries. Among these structures studied, Utrn‐B protein was abundant in brain capillaries as compared with blood vessels and choroid plexus. Histograms show means ± SD. Representative blots from a minimum of five experiments. Lower lanes show blots probed with tubulin antibodies. I,J. Histogram of densitometric quantification of Utrn‐A and Utrn‐B proteins in choroid plexus, blood vessels and brain capillaries normalized by corresponding tubulin bands, respectively showing significantly higher amount of Utrn‐A in choroid plexus than brain vascular elements, whereas Utrn‐B was significantly higher in brain capillaries compared with choroid plexus and blood vessels. Compared with Utrn‐A and Utrn‐B protein levels in the choroid plexus; statistical analysis was performed by one‐way analysis of variance (n = 6; *P < 0.05, **P < 0.001). Scale bar, 50 µm.
