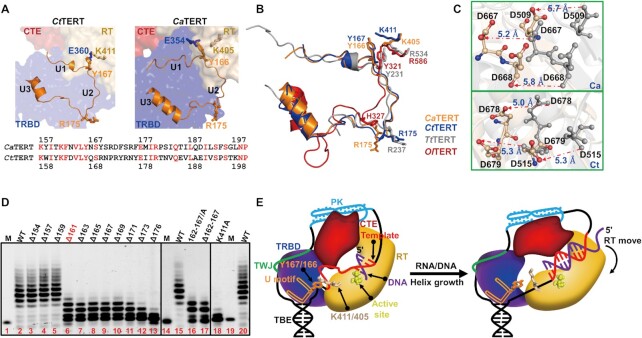
Figure 5.
Newly identified U-motif structure and function. (A) The identified U-motif conformation and key residues in CtTERT (left) and CaTERT (right). The detailed interactions are shown in Supplementary Figure S12. The residues of U-motif from CaTERT and CtTERT are shown below the figure. (B) Superimposition of the identified U-motif structures in CaTERT (orange), CtTERT (blue), TtTERT (gray, PDB: 6D6V), and OlTERT (red, PDB: 4O26). (C) Significant movement of the key residues in the active site of CaTERT (above) and that of CtTERT (bottom) in the presence of the integral U-motif (gray) and partially truncated U-motif proteins (wheat). Additional conformational changes and the detailed interactions are shown in Supplementary Figure S16B-C. (D) Telomerase activity assays performed with a series N-terminally truncated or structure-guided point mutants from CtTERT. WT is CtTERTFL, Δ154 means residues 1–154 are deleted, namely CtTERT155–879, and others are similar to this; 162–167/A and Δ162–167 mean residues from 162 to 167 either systematically substituted by alanine or entirely deleted on the basis of the full-length CtTERT; K411A means residue K411 substituted by alanine, namely CtTERT1–879/K411A. The experiments were carried out under experimental conditions as described in Figure 4D. (E) Proposed model for the U-motif-mediated conformational change upon the completion of telomeric synthesis. The RNA template is bound between the TBE stem at its 5′-end and a PK motif at its 3′-end. Similarly, as observed in the TtTERT-TER complex structure determined by Cryo-EM, TER embraces the TERT. While the spatial positions of the TBE and PK are mobilized by the U-motif and other regions of TERT, the single stranded RNA template is flexible prior to replication (left). However, as the RNA template is replicated, the RNA/DNA hybrid becomes shorter than the ssRNA. The produced tension rotates the global conformation between the TRBD and RT domain and consequently disrupts the cation-π interaction between Y167/166 and K411/405 (right), which is essential for maintaining TERT in an active conformation.