Fig. 4.
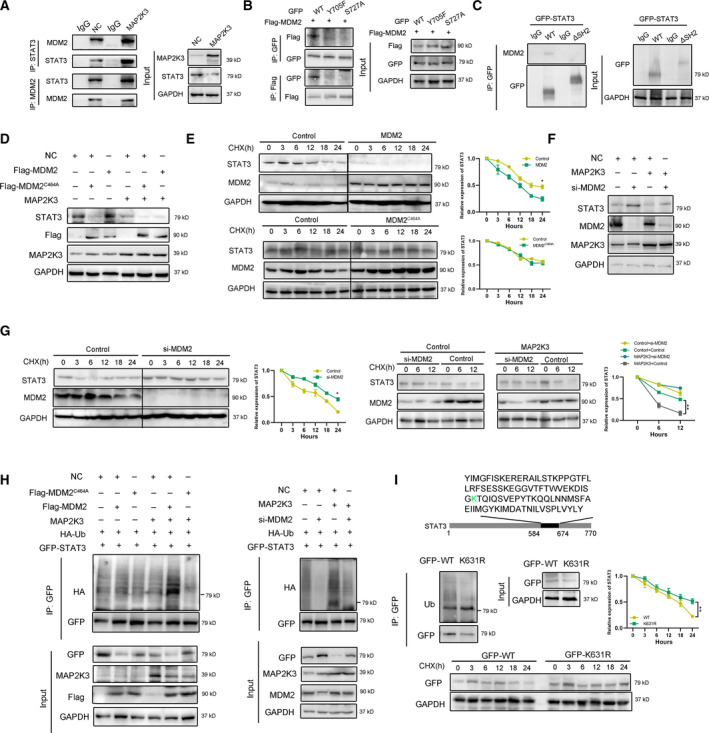
MAP2K3 interacted with E3 ligase MDM2 to promote STAT3 degradation. (A) The interaction of STAT3, MAP2K3, and MDM2 was detected by co‐immunoprecipitation in KYSE150 cells. (B) The interaction of GFP‐tagged STAT3 wild‐type (WT), Y705F, S727A, and Flag‐MDM2 was detected by co‐immunoprecipitation in 293T cells. (C) The interaction of GFP‐tagged STAT3 wild‐type (WT) or SH2 domain deletion (ΔSH2) and MDM2 was detected by co‐immunoprecipitation in KYSE150 cells. (D) MDM2 decreased STAT3 protein. KYSE150 cells were transfected with Flag‐MDM2 or Flag‐MDM2C464A as well as control or MAP2K3 transfection. The protein expression level of STAT3 was assayed by western blot. (E) The cells expressing MDM2 or MDM2C464A were treated with cycloheximide (CHX, 200 µg·mL−1). The protein levels of STAT3 and MDM5 were analyzed by western blot. (F) Knockdown MDM2 increased STAT3 protein. KYSE150 cells were transfected with si‐MDM2 as well as control or MAP2K3 transfection. (G) KYSE150 cells were transfected with control or MDM2 siRNAs treated with CHX, and the protein levels of STAT3 and MDM2 were analyzed by western blot. (H) MDM2 ubiquitylates STAT3. KYSE150 cells were transfected with indicated plasmids or siRNA for 48 h. (I) KYSE150 cells were transfected with indicated plasmid, and western blot was performed to analyze the expression of indicated proteins and ubiquitination. Error bars represent the SD from at least three independent biological replicates. (*P < 0.05; **P < 0.01; ***P < 0.001 by Student’s t‐test).
