Figure 6.
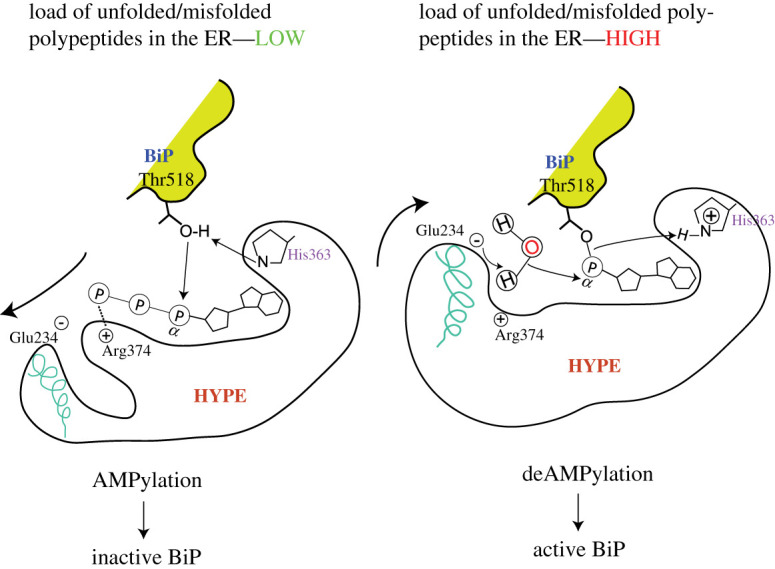
HYPE switching between AMPylation and deAMPylation states in response to ER conditions. HYPE AMPylates BiP when the unfolded protein load in the ER is low and deAMPylates BiP under conditions of stress that often increase unfolded protein load. AMPylated BiP cannot function as a chaperone and is pooled into a reservoir of non-functional BiP that can be activated under conditions of stress by deAMPylation. It is currently hypothesized that Glu234 mediates the switch between AMPylation and deAMPylation competent HYPE conformations. The bold arrows indicate the movement of α-inh that harbours Glu234. During AMPylation, Glu234 disengages from the catalytic site and allows the alignment of key residues in the catalytic FIC motif and Thr518 of BiP. When cells require functional BiP to tackle increasing loads of unfolded polypeptides, Glu234 engages with Arg374 and coordinates the attack of a water molecule (acting as a nucleophile) on the bond between Thr518 and AMP. The smaller arrows indicate a hypothetical electron transfer between various moieties involved in the proposed catalytic mechanism. This figure is adapted from Preissler et al. [77].
