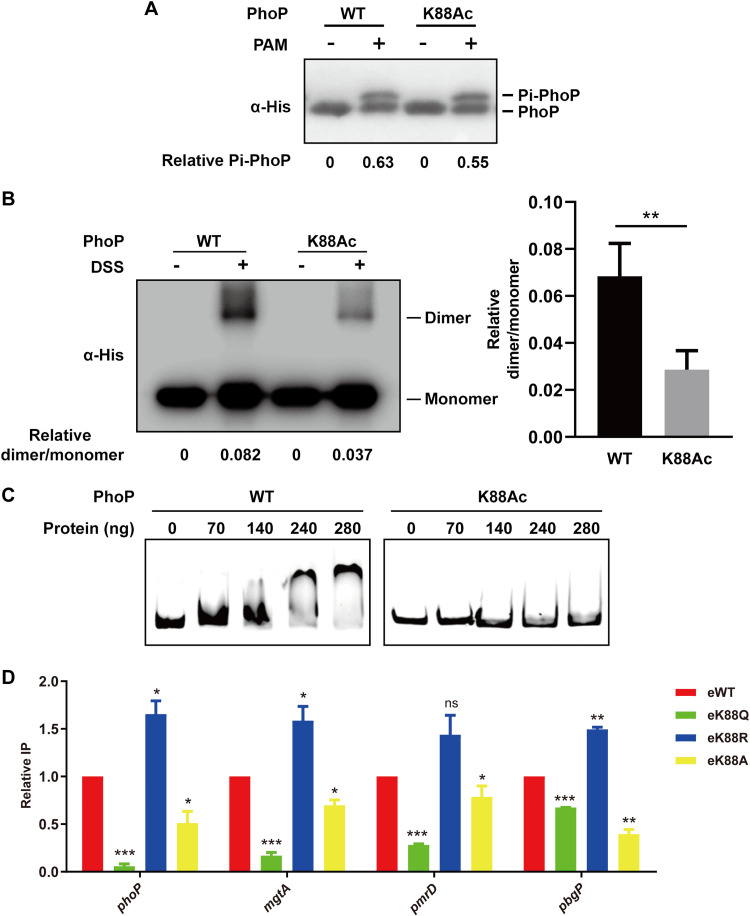
FIG 3.
Acetylation of K88 impairs the dimer formation and DNA-binding ability of PhoP. (A) The phosphorylation of PhoP K88Ac in vitro. Purified wild-type PhoP and PhoP K88Ac proteins were incubated with or without 20 mM PAM, respectively. The samples were resolved on 12% SDS-PAGE gel containing Phos-tag and analyzed with anti-His antibody. Representative results from three independent experiments of Western blots are shown. (B) Dimer-formation of PhoP K88Ac. Purified wild-type PhoP or K88Ac was subjected to cross-linking with DSS. Western blots were probed with anti-His antibody and independently repeated at least three times. The relative ratio of dimer over monomer of PhoP was quantified. Error bars indicate ± SDs of triplicate experiments; **, P < 0.01. Student’s t test. (C) DNA-binding activity of PhoP K88Ac assessed by EMSA. 6′-FAM-labeled phoP promoter was incubated with His-tagged wild-type PhoP or PhoP K88Ac at the indicated concentration, and then the reaction products were analyzed in 5% PAGE. Representative EMSA results from three independent experiments are shown. (D) DNA-binding activity of PhoP K88 derivative mutants compared to WT in log-phase acid tolerance response by ChIP. eWT or phoP K88 mutant strains in which phoP was flag-tagged in chromosome were cultured in EG medium at pH 5.0 for 1 h and harvested. The total cell lysate was immunoprecipitated by anti-Flag antibody. The candidate gene promoter before (input) and after (Flag-ChIP) were quantified by qPCR and represented as the relative ratio of ChIP/input. Results are shown as mean ± SD from three independent experiments; *, P < 0.05; **, P < 0.01; ***, P < 0.001. ns, no significance. Student’s t test.