Figure 3. Dysregulated interplay between DNA and histone methylation in human OGID syndromes.
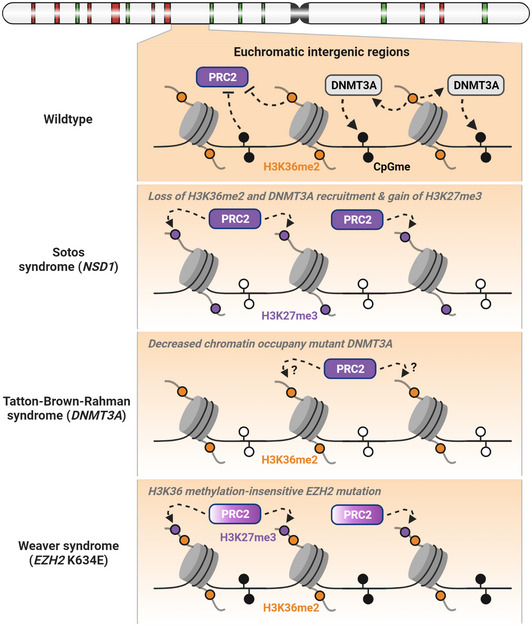
During normal development, H3K36me2 facilitates the deposition of CpG methylation by recruiting DNMT3A at euchromatic intergenic regions, and these two modifications act together to antagonize PRC2 and H3K27me3. In Soto syndrome, NSD1 mutations and deletions lead to reduced H3K36me2 and CpG methylation, and a resulting gain of H3K27me3. In TBRS, loss‐of‐function mutations in DNMT3A reduce CpG methylation, although its impact on H3K36me2 and H3K27me3 is unclear. Some Weaver syndrome patients carry missense mutation of EZH2 (e.g., K634E) that renders the PRC2 insensitive to inhibition by H3K36 methylation, which could potentially leads to accumulation of H3K27me3 at intergenic regions despite the presence of H3K36me2. The significant overlap in clinical features of Soto, Weaver, and TRBS patients suggests that an imbalance of H3K36me2, H3K27me3, and CpG methylation could represent a common pathogenic mechanism.
