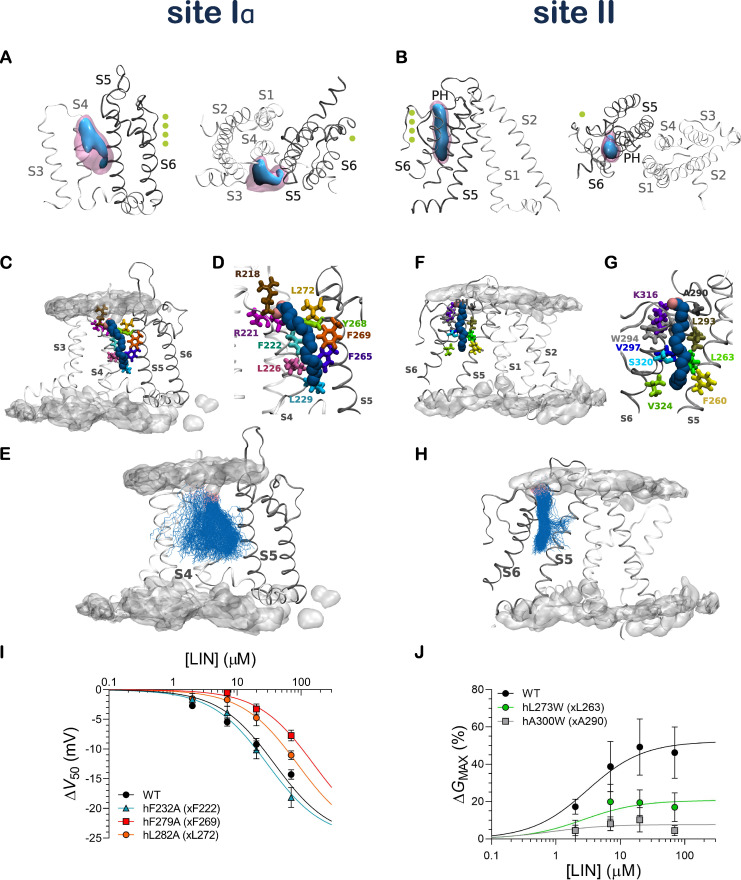
Figure 4.
LIN binds at interaction sites through a network of charged and hydrophobic residues. (A and B) 3-D contour maps of the occupancy of a LIN constant binder at site Iα (A) and site II (B) are shown from side view (left) and top view (right). Threshold values are set at 10% (blue) and 30% (pink) of the total time. PH denotes pore-helix. Potassium ions in the permeation pathway are indicated as green spheres. (C and D) Side view of a LIN constant binder interacting with the voltage-sensing domain at site Iα. (E) Overlay of all LIN constant binder poses at site Iα. The LIN head group is shown in pink and the LIN tail in blue. (F and G) Side view of a LIN constant binder interacting with the pore domain at site II. (H) Overlay of all LIN binder poses at site II. The occupancy of the membrane lipid head groups is shown for visual purposes to denote the position of the membrane bilayer. (I and J) Mutation of predicted binder residues impairs the effect of LIN. (I) Concentration dependence of the LIN effect on V50 of indicated hKCNQ1 site I mutants. Data shown as mean ± SEM; n = 4–8 per data point. Concentration-response curves were fitted using Eq. 2 with the Hill coefficient constrained to −1 (see Materials and methods for details). ΔV50,max was constrained to −25 mV to make the fits more robust. (J) Concentration dependence of the LIN effect on Gmax of indicated hKCNQ1 site II mutants. Data shown as mean ± SEM; n = 6–8 per data point. Concentration-response curves were fitted using Eq. 2 with the Hill coefficient constrained to 1 (see Materials and methods for details). ΔV50,max denotes maximal effect in ΔV50.