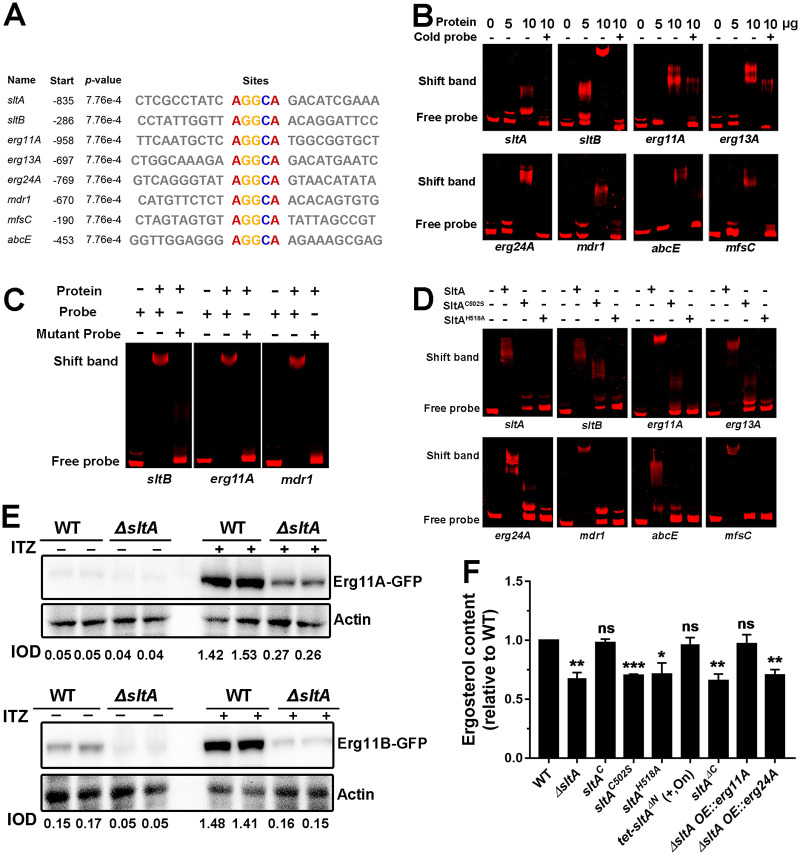
FIG 6.
SltA binds to the AGGCA motif in the promoters of ergosterol biosynthesis and drug pump genes and affects related gene expression and function. (A) The promoters of the indicated genes contain a conserved AGGCA motif. The “Start” column shows the number of base pairs upstream of ATG. (B) EMSA showed in vitro binding of the indicated amounts of purified SltA protein to Cy5-labeled promoter fragments (20 ng) of indicated genes. We used 100× nonlabeled DNA fragments (cold probe, 2 μg) as competition assays. (C) An EMSA with a mutated binding motif. The consensus binding motif AGGCA from the promoters of the sltB, erg11A, and mdr1 genes was mutated into CAAAC and used for EMSA analyses. (D) EMSA showed in vitro binding of the purified SltA, SltAC502S, and SltAH518A proteins (10 μg) to Cy5-labeled promoter fragments (20 ng) of the indicated genes. (E) Western blot analysis for the expression of Erg11A and Erg11B in the parental wild-type and ΔsltA mutant strains with or without 0.01 μg ml−1 of itraconazole. The protein actin was used as a loading control. Each protein was loaded in two replicates. The value of integrated option density (IOD; IODtarget protein/IODactin) measured by Image-Pro Plus (IPP) showed the relative protein quantity. (F) The same number of spores (2 × 107) from the indicated strains grown for 24 h at 37°C in MM with 0.5 mM calcium and 0.015 μg ml−1 of itraconazole for subsequent ergosterol measurement by HPLC. The ergosterol content of each mutant was normalized to that of the parental wild-type strain and is shown as relative fold change. Samples were assessed in biological triplicates. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, P > 0.05 according to Student’s t test with Welch’s correction. +, strains were grown on medium with 5 mg liter−1 of doxycycline. tet-sltAΔN was induced (“on” state) in the presence of doxycycline.