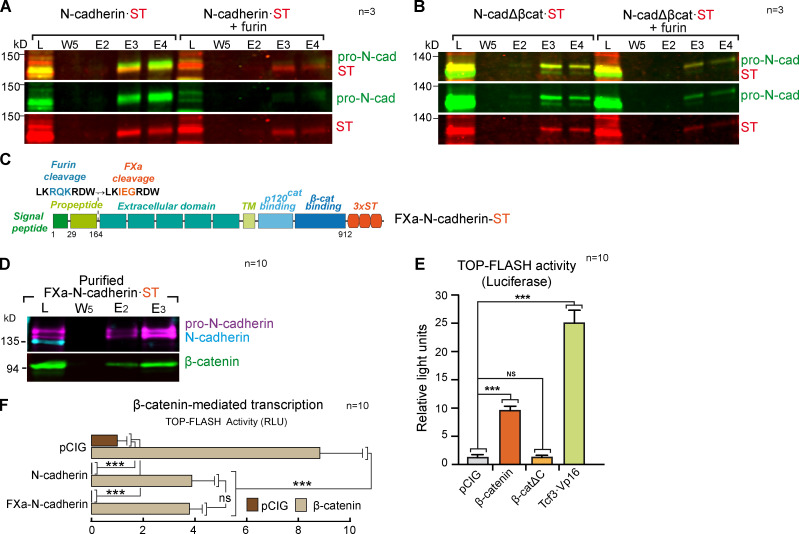
Figure S3.
The β-catenin–binding domain of N-cadherin is required for furin-mediated propeptide cleavage. (A) Western blot showing Strep-Tactin–purified fractions of HEK-293 cultures transfected with a control vector or a vector expressing furin and N-cadherin·ST. The blot was probed with antibodies against pro-N-cadherin (green) and ST (red); n = 3. (B) As in A, but with N-cadherinΔβcat. (C) Scheme of the FXa-N-cadherin·ST construct in which the furin cleavage site on N-cadherin has been substituted with an FXa site similar in size but not cleavable in the cell (n = 3). (D) Western blot showing Strep-Tactin–purified fractions of HH18 chicken NTs transfected with FXa-N-cadherin·ST and probed with antibodies against pro-N-cadherin (purple), N-cadherin (cyan), and β-catenin (green). Note that FXa-N-cadherin·ST does not generate mature N-cadherin (n = 10). (E) Wnt pathway activity (TOPFlash) studied with a luciferase reporter assay in HH18 chicken NTs 24 hpe with control, β-catenin, β-cateninΔC, β-catenin, or Tcf3·Vp16. Bar graphs show the mean ± SD. Each experimental condition was compared with every other experimental condition using a one-way ANOVA with Tukey’s multiple comparisons test (n = 10). (F) The bar graph represents the mean ± SD (three independent experiments) of luciferase reporter assays using TOPFlash vector 48 hpe with pCIG, N-cadherin, or FXa-N-cadherin cotransfected with β-catenin or not. Each experimental condition was compared with every other experimental condition using a one-way ANOVA with Tukey’s multiple comparisons test (n = 10). ***, P < 0.001. cad, cadherin; E, elution; L, lysate; RLU, relative light unit; TM, transmembrane domain; W, wash.