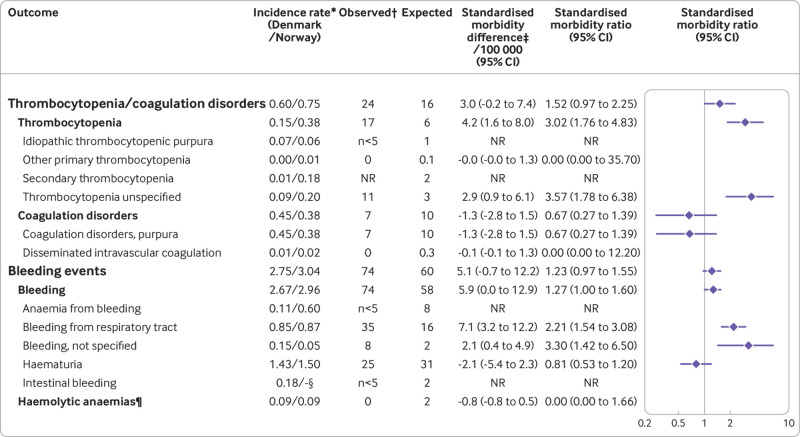
Fig 2.
General population incidence rates, observed and expected counts of events, excess events per 100 000 vaccinations, and standardised morbidity ratios of thrombocytopenia/coagulation disorders and bleeding events within 28 days of vaccination in a cohort of 18-65 year old Danish and Norwegian people (n=281 264) receiving their first dose of the Oxford-AstraZeneca covid-19 vaccine (ChAdOx1-S). NR=not reported owing to privacy regulations. *Per 1000 person years in the general population. †Observed events are not mutually exclusive (ie, one patient can contribute to two different third level outcomes. However, two different third level outcomes would only count once towards a common second level outcome, and similarly only once in a first level outcome). ‡Expected events based on incidence rates in the general population. §Not available in the Norwegian data source. ¶Including haemolytic anaemia, haemolytic uraemic syndrome, and paroxysmal nocturnal haemoglobinuria