Figure 2. Clinical Remission at 30 Weeks (Primary Outcome).
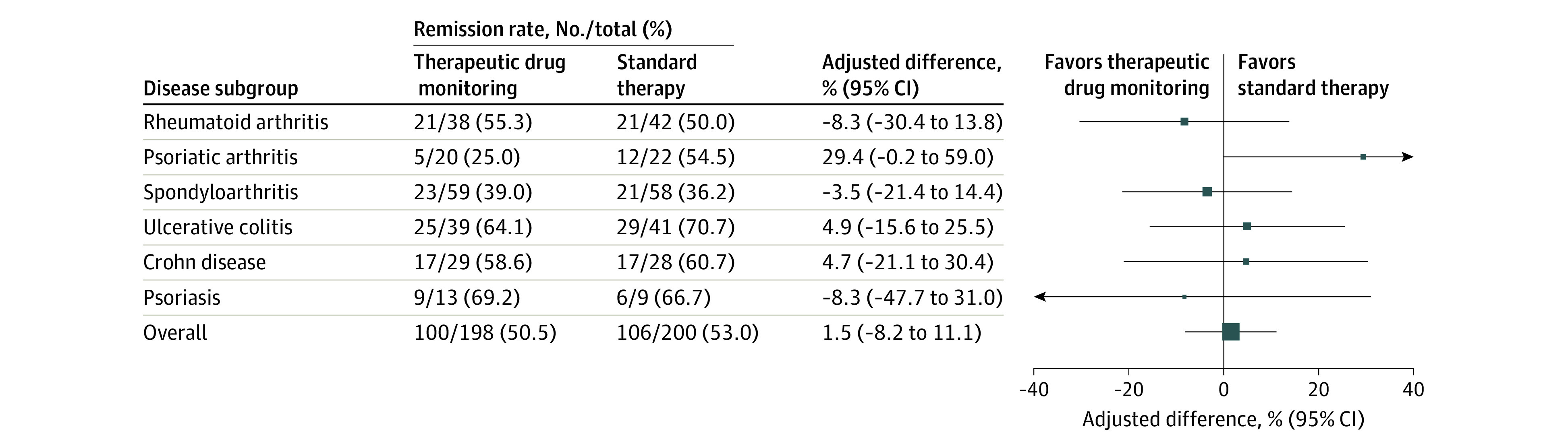
Adjusted difference in clinical remission rate at 30 weeks overall (the primary end point) and by disease subgroup. The adjusted difference in remission rate was assessed by mixed-effects logistic regression using data from all patients exposed to the randomized intervention (patients having received the second infusion with a recorded treatment decision for the third infusion). Size of data markers is proportional to the number of patients in the group. Clinical remission was defined by disease-specific composite scores: a Disease Activity Score in 28 Joints lower than 2.6 in patients with rheumatoid arthritis and psoriatic arthritis, an Ankylosing Spondylitis Disease Activity Score lower than 1.3 in patients with spondyloarthritis, a Partial Mayo Score of 2 or lower with no subscores greater than 1 in patients with ulcerative colitis, a Harvey-Bradshaw Index of 4 or lower in patients with Crohn disease, and a Psoriasis Area and Severity Index of 4 or lower in patients with psoriasis. See the Box and eTable 3 in Supplement 1 for detailed descriptions of the Disease Activity Score in 28 Joints, Ankylosing Spondylitis Disease Activity Score, Partial Mayo Score, Harvey-Bradshaw Index, and Psoriasis Area and Severity Index.
