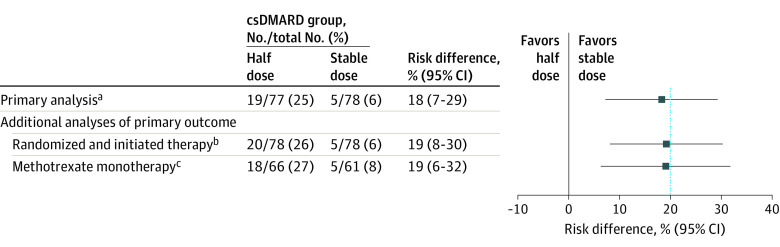
Figure 2. Flare Rate Within 12 Months (Primary Outcome) in Half-Dose vs Stable-Dose Antirheumatic Drug Treatment.
Flare was defined as a combination of Disease Activity Score (DAS) above the cutoff for remission (1.6), a change in DAS of at least 0.6, and at least 2 swollen joints or that both the treating physician and the patient agreed that a clinically significant flare had occurred. The blue, dotted, vertical line represents the noninferiority margin. csDMARD indicates conventional synthetic disease-modifying antirheumatic drug.
aThe primary analysis was performed in all randomized patients meeting the study entry criteria and with no protocol deviations affecting the treatment efficacy (defined as failure to follow the treatment regimen or withdrawal from the study).
bFour patients who were randomized but did not have verified initiation of treatment are excluded (2 from each group).
cAnalysis performed in patients within the primary analysis population who used methotrexate monotherapy.