Fig. 1. Radiographic responses from a phase 2 study of anti–PD-1 responder–derived FMT and pembrolizumab in PD-1–refractory melanoma.
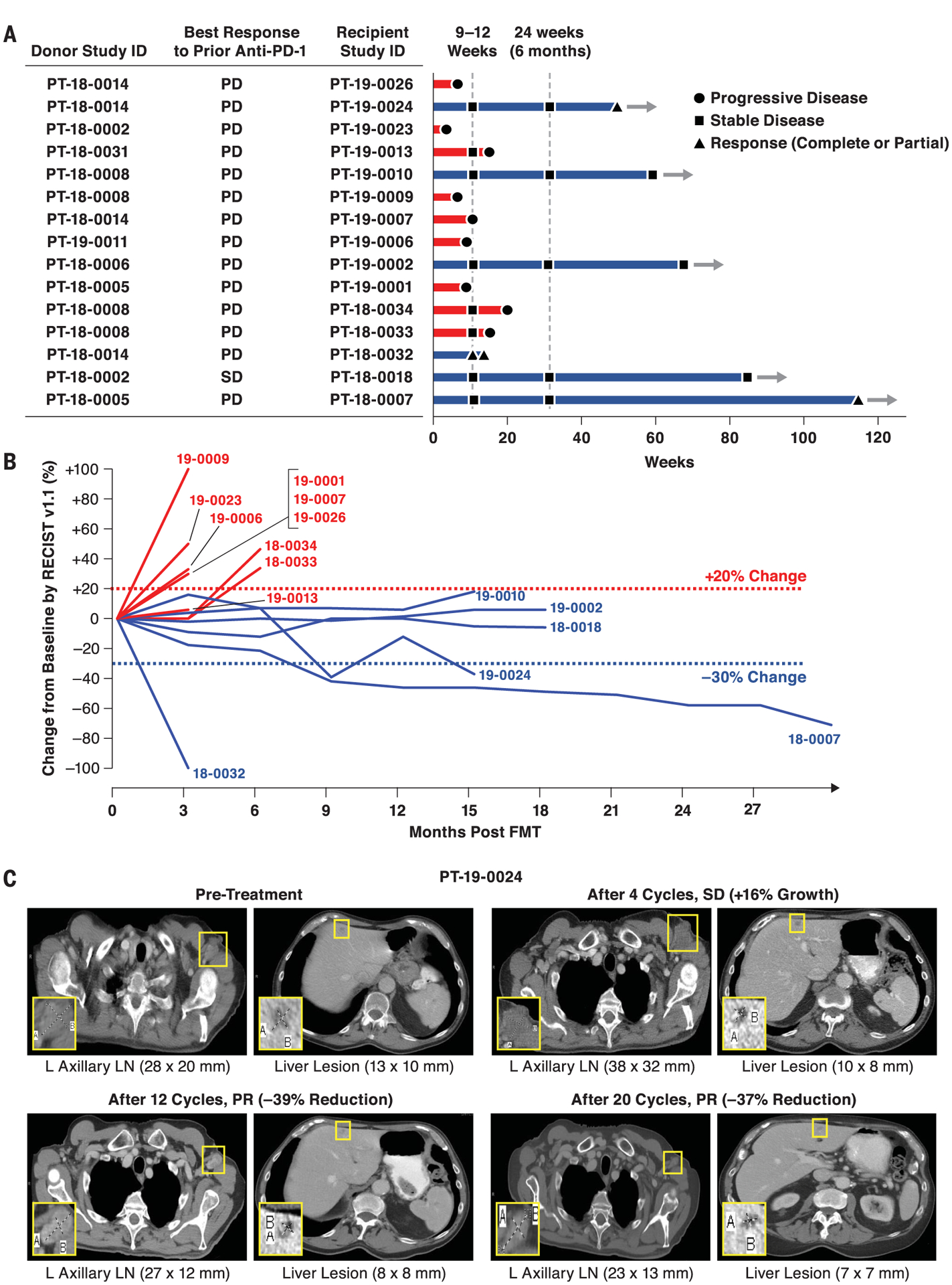
Melanoma patients who had primary refractory disease to anti–PD-1 therapy received FMT derived from individual melanoma patients with durable OR to anti–PD-1 therapy. FMT was administered colonoscopically on day 0 along with pembrolizumab (200 mg). Pembrolizumab was repeated every 3 weeks. Restaging scans were performed at weeks 9 to 12 and repeated every 9 to 12 weeks while in the study. Patients remained in the study until intolerable toxicity, RECIST v1.1–confirmed disease progression, or completion of 35 cycles of pembrolizumab. (A and B) Treatment exposure and response duration by RECIST v1.1 (investigator assessed; n = 15). (A) FMT donor and best response to prior line(s) of anti–PD-(L)1 therapy singly or in combination are shown for each FMT-recipient patient. The length of each bar corresponds to the duration of time that patients received treatment (in weeks). Response status is color coded (R, blue; NR, red). Response symbols represent status at first restaging scan (9 to 12 weeks) and at most recent review. Patients with ongoing response in the study are depicted with horizontal arrows. (B) Radiographic change of tumor burden from baseline (investigator assessed per RECIST v1.1; n = 15). One patient (PT-18–0018) had initial disease stability with subsequent progression after antibiotic therapy and was offered a retransplant with the same donor, with subsequent disease stabilization. (C) Representative CT scans from one responding patient. CT scans from patient PT-19–0024 at four separate time points depict initial tumor growth after FMT followed by eventual PR. L, left; LN, lymph node.
