Figure 5. Increased H3K27 and H3K9 acetylation enhances Aβ42 toxicity in Drosophila.
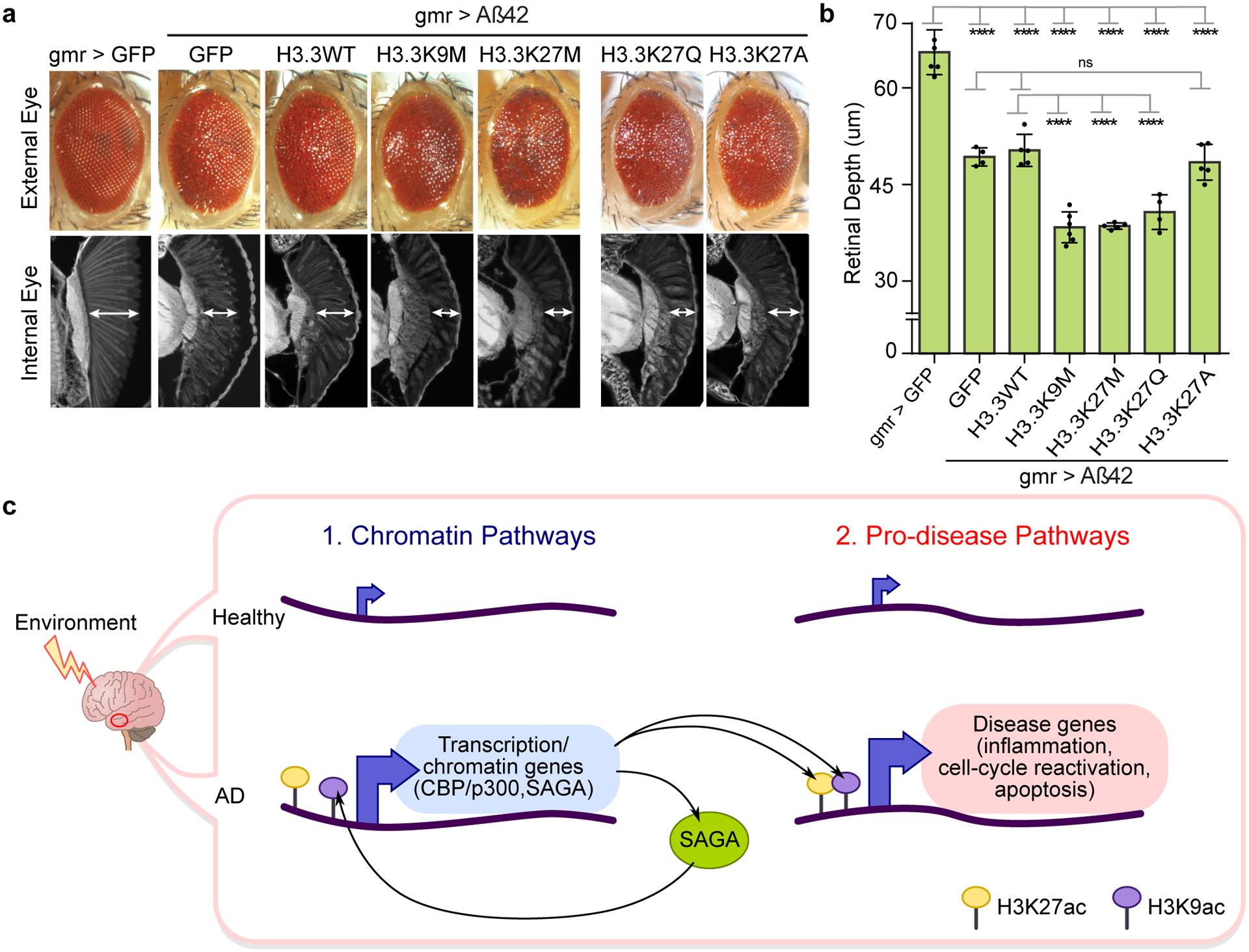
(a) Drosophila eye showing that histone mutants H3.3K9M (lysine (K) to methionine (M) mutation at residue 9), H3.3K27M (K to M mutation at residue 27) and H3.3K27Q (K to glutamine (Q) mutation at residue 27) independently enhance Aβ42 toxicity both in external (top) and internal eye tissue (bottom). Histone mutant H3.3K27A (K to alanine (A) mutation at residue 27) has no effect on Aβ42 toxicity. Expression of H3.3K27M and HK9M globally increase H3K27ac91, while H3.3K27Q mimics acetylation93, and H3.3K27A mimics absence of acetylation (Supplementary Table 14 for fly genotypes). (b) Barplot (with individual data points) represents mean ± SD of internal retinal depth in N = 4–6 individual animals per genotype (**** P < 0.0001, 1-way ANOVA (F (6, 28) = 83.24) with Tukey’s multiple comparisons test. Aß42+GFP vs: Aß42+H3.3WT (P = 0.9962); Aß42+H3.3K27A (P = 0.9987). Aß42+H3.3WT vs: Aß42+H3.3K9M (P = 2.3771 × 10−7); Aß42+H3.3K27M (P = 7.8390 × 10−7); Aß42+H3.3K27Q (P = 6.9131 × 10−5)). (c) Model of aberrant activation of chromatin and pro-disease pathways in AD. Increased H3K27ac and H3K9ac (by CBP/p300 and SAGA) drive activation of chromatin (left) and pro-disease pathways (right) in AD. Increased expression of transcription and chromatin genes (left), including CBP/p300 and TRAPP in the SAGA complex (left), may be upstream and reinforce activation of pro-disease pathways. H3K9ac at CREBBP (potentially by SAGA) leads to a positive feedback loop of sustained CBP expression and downstream histone acetylation in the AD epigenome. Environmental stressors may be upstream the activation of chromatin pathways.
