Figure 1.
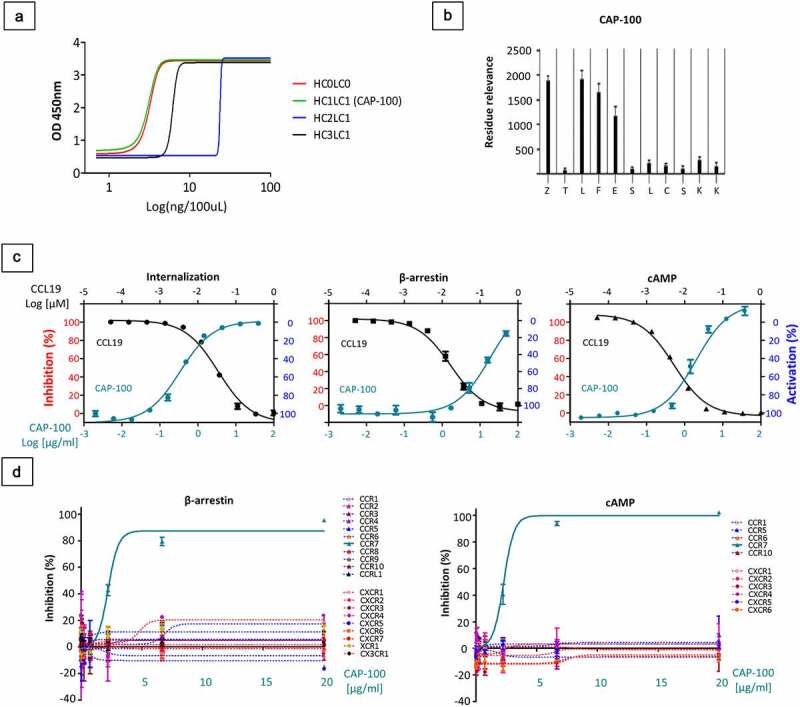
CAP-100 specifically blocks CCR7-pathways with high affinity and selectivity. A) Concentration dependent binding of humanized-729 candidates to 100 ng of CCR7 immunogen SYM1899, the sulfated peptide derived from the N-terminus of human CCR7. The graph shows the OD 450 nm measured by ELISA of 1:1 serial dilutions of antibody to a minimum concentration of 0.0078 μg/ml. B) Epitope mapping of CAP-100 mAb. Peptides were generated bearing single amino acid substitutions at each position of the wild-type sequence. Values of binding to each peptide were assayed by ELISA (residue relevance). The graph shows the OD-405 nm measurements. At the bottom of the plot the native sequence is indicated. Z, sulfated Tyr. C) CAP-100 inhibits CCL19-induced CCR7 internalization, β-arrestin recruitment, or Gi-mediated cAMP reduction. The percentage of inhibition was determined in CCR7+ CHO-K1 cells incubated with CAP-100 antibody and EC80 CCL19. The concentration of CAP-100 [μg/mL] and CCL19 [nM] are plotted on the x-axes versus inhibition (left y-axis) or activation (right y-axis). Data were normalized to the minimal and maximal response observed in the presence of vehicle and EC80 CCL19. The mean ± SD of triplicates is shown. D) CAP-100 mediates specific neutralization of CCR7. Selective inhibition by CAP-100 was evaluated in β-arrestin and cAMP assays in a panel of 20 chemokine receptors stably expressed by CHO-K1 cells. The following pairs of ligand/receptor were included: CCL3/CCR1; CCL27/CCR10; CCL2/CCR2; CCL13/CCR3; CCL22/CCR4; CCL3/CCR5; CCL20/CCR6; CCL1/CCR8; CCL25/CCR9; CCL19/CCR7; CCL19/CCRL1; fractalkine/CX3CR1; CXCL8/CXCR1; CXCL8/CXCL2; CXCL11/CXCR3; CXCL12/CXCR4; CXCL13/CXCR5; CXCL16/CXCR6; CXCL12/CXCR7; Lymphotactin/XCR1. The mean ± SD of triplicates is shown
