Abstract
Background and Objectives:
Rapid on-site cytologic evaluation (ROSE) increases the diagnostic yield of EUS-FNA. However, ROSE requires the presence of a cytopathologist and additional cost and time for slide staining and interpretation. Macroscopic on-site examination (MOSE) was recently introduced as an alternative to ROSE and showed high accuracy for the use in pathologic diagnosis. We evaluated the efficacy of MOSE in terms of tissue acquisition and diagnostic accuracy for abdominal lesions.
Methods:
We analyzed consecutive patients included who underwent EUS-guided fine needle biopsy (FNB) between January 2019 and November 2019. All procedures were done by dry suction using a 22G needle. Obtained specimens were expelled onto filter papers and evaluated by MOSE. Needle pass was done until the acquisition of satisfactory whitish macroscopic visible core or bloody tissue granules. The primary outcomes were successful tissue acquisition and accuracy for pathologic diagnosis.
Results:
In 75 patients (male, 52%; median age: 62 years), the pancreas was the most commonly targeted organ (81.4%) and the median target diameter was 25 mm. The median number of needle passes was 2.0 (range, 2–5). Successful targeting of the lesion was achieved in 75 patients (100%) and overall accuracy was 97.3%. There were no procedure-related adverse events.
Conclusions:
MOSE was effective for complementing EUS-FNB by ensuring the adequate acquisition of biopsy specimens with a minimal number of needle passes while providing a critically high diagnostic accuracy. MOSE seems to be a viable alternative to ROSE in select clinical situations.
Keywords: EUS, fine needle biopsy, macroscopic on.site examination
INTRODUCTION
EUS-FNA opened a new era in the diagnostic field of gastrointestinal pathology[1] and has been proven valuable for various lesions in the pancreas, liver, spleen, and intra-abdominal lymph nodes.[2] Despite its efficacy, however, the diagnostic accuracy of EUS-FNA ranged from 55% to 100% in previous studies depending on the disease characteristics and locations.[3,4,5] Moreover, tissue acquisition in EUS-FNA is often hindered by indirect targeting under EUS, small needle caliber, lack of endosonographer's experience, and difficult locations of the target.[3,6] To overcome these limitations, various efforts were made through the adaptation of sampling techniques, renovation of the shape and size of the needle, cytopathologic evaluation, and sampling processing.[1] Rapid on-site evaluation (ROSE), which refers to the immediate cytologic assessment after FNA by a cytopathologist, was found to be useful in increasing the adequacy of sample acquisition and reducing the number of needle passes in EUS-FNA.[7,8] ROSE is thus expected to shorten the time needed for the diagnosis and decrease the occurrence of procedure-related complications and has shown its efficacy in two meta-analyses.[7,8]
However, ROSE failed to show a significant benefit in another two meta-analyses,[9,10] and its efficacy is still under debate.[11] A randomized trial reported no significant difference in the diagnostic yield of malignancy, proportion of inadequate specimens, accuracy, overall procedure time, adverse events, number of repeat procedures, and costs according to ROSE.[12] Diagnostic yield and accuracy did not significantly differ even for lymph node lesions in another randomized trial.[13] Furthermore, ROSE requires the presence of a cytopathologist as well as higher cost and additional time for staining and slide interpretation and is thus not readily available;[14] accordingly, a global survey showed that ROSE was available in most of the USA-based centers (98%) but only in about half of the centers based in other regions (Europe, 48% and Asia, 55%).[14] In the early era of EUS-FNA, ROSE was considered necessary considering the expense of the examination and the time consumed during multiple needle passes;[15,16] however, due to the development of techniques for FNB, much more tissue can be obtained with a smaller number of needle passes.[17,18] Importantly, Mohamadnejad et al. showed that needle pass number beyond four did not significantly increase sensitivity for detecting pancreatic malignancy in EUS-guided FNA; therefore, using ROSE for reducing the number of needle passes would be needless in the era of FNB,[19] and ROSE may not confer significant additive value for experts.[9]
Macroscopic on-site evaluation (MOSE) of the biopsy specimen by an endoscopist was recently introduced as an alternative to ROSE[20,21,22] and showed promising results. Iwashita et al. used a 19 G needle by suction method and the endosonographers decided the number of FNA passes with MOSE; the number of needle passes was 2 or 3 in all patients, and the overall accuracy was 95.5%.[20] Ki et al. also performed MOSE by using a 22 G needle without suction, and 93% required only one needle pass without ROSE while providing an overall diagnostic accuracy of 94% and diagnostic adequacy of 98%. Recent, large randomized controlled trial showed MOSE showed fewer needle pass with comparable diagnostic yield to conventional method.[23] In this study, we aimed to determine the efficacy of MOSE in terms of tissue acquisition and accuracy for providing oncologic diagnosis in abdominal lesions.
METHODS
Patients
We analyzed adult patients (age ≥20 years) who underwent EUS-guided FNB between January 2019 and November 2019 at Asan Medical Center (Seoul, Korea) for solid lesion, cystic lesion, and lymph nodes. Imaging studies (i.e., computed tomography or magnetic resonance imaging) were performed before the procedure. The exclusion criteria were pregnancy, severe coagulopathy (international normalized ratio >1.5), or thrombocytopenia (platelet count <50,000). All patients had been prospectively included, and the data were retrospectively analyzed in this study. This study was approved by the Institutional Review Board of Asan Medical Center (approval no. 2019-1689).
Procedures
All procedures were performed done by single expert echoendoscopist (D. W. S) under sedation with intravenous midazolam by using a conventional linear array echoendoscope (GF-UCT 260; Olympus Optical, Tokyo, Japan) and 22 G needles (Acquire; Boston Scientific, Natick, MA, USA) for tissue acquisition. After EUS evaluation of the target lesion, FNB was performed. The needle was inserted into the target tissue under EUS guidance, and once the lesion was penetrated, the stylet was removed, and negative pressure using a 10 mL syringe was applied. Specimens were obtained by moving the needle back and forth, and the suction was released before removing the needle. By reinserting the stylet, the biopsy specimens were expelled onto a small piece of filter paper that facilitates macroscopic evaluation by absorbing the surrounding blood. The minimum number of needle passes was set at 2. After macroscopic evaluation of the biopsy specimen by an endoscopist, additional needle pass was performed until satisfactory achievement of whitish macroscopic visible core (MVC) 0 or bloody tissue granules [Figure 1]. MOSE was performed immediately after every needle pass. If there was whitish MVC either from two samples or both, the procedure was stopped and the sample was sent to the pathology department. In case of bloody tissue granules, additional needle passes were performed until the two samples of bloody tissue granules were obtained [Figure 2].
Figure 1.
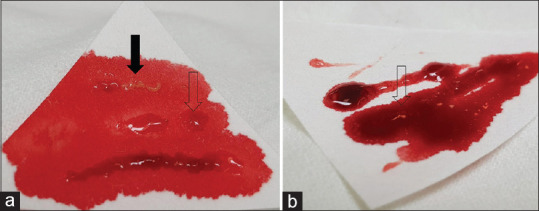
Representative pictures of the obtained tissue. Whitish macroscopic visible tissue is a macroscopically visible whitish and nonbloody core tissue (a, black arrow) bloody tissue granules is not a core tissue and not clearly visible, but tiny spots in the bloody background could be seen (b, open arrow)
Figure 2.
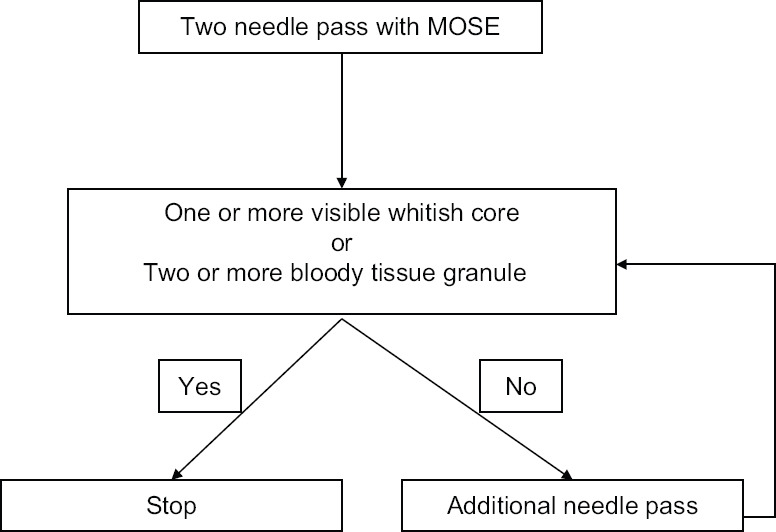
Study method by macroscopic on-site evaluation
Outcomes
The primary outcomes were technical success and diagnostic accuracy by tissue acquisition with MOSE for pathologic diagnosis. Technical success was defined as successful puncture of the lesion with successful achievement of the specimen according to the study design (i.e., whitish MVC or blood tissue granule). Diagnostic accuracy was defined as (correct diagnosis)/all cases. Final diagnoses were made based on either surgical pathology or clinical course with radiologic findings in benigncases.
Statistical analysis
The baseline characteristics and clinical outcomes of the patients are presented as median, range, or percentage as appropriate. Data were analyzed using R v3.5.3. (R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org).
RESULTS
Patients and lesion characteristics
A total of 75 patients (median age: 62 (range, 17–84); male, 52%) were included. The most common location of the target was the pancreas (n = 61), followed by lymph nodes (n = 6) and the liver (n = 3). The median size of the lesions was 25 mm (range, 8–90). Among 75 lesions, 67 were sold lesions and 8 lesions were cystic. Baseline characteristics are summarized in Table 1.
Table 1.
Baseline characteristics of the patients and targeted lesion
| Variables | Total (n=75) |
|---|---|
| Age, years (range) | 62 (17-84) |
| Male:female | 39:36 |
| Solid:cyst | 67:8 |
| Diameter, mm (range) | 25 (8-90) |
| Location, n (%) | |
| Pancreas | 61 (81.4) |
| Lymph node | 6 (8.0) |
| Liver | 3 (4.0) |
| Intra-abdominal soft tissue | 2 (2.7) |
| Common bile duct | 1 (1.3) |
| Adrenal gland | 1 (1.3) |
| Duodenum | 1 (1.3) |
Outcomes
All 75 (100%) patients had technical success in targeting of the lesion. The median number of needle passes was 2.0 (range, 2–5) for obtaining the adequate amount of tissue by MOSE. Four (5.3%) patients required more than “4” passes for acquisition of satisfactory MCV or bloody tissue granules.
The final diagnosis was malignancy in 30 patients and benign lesions in 45 patients. The overall accuracy with MOSE was 97.3% (73/75); sensitivity 96.7%, specificity 97.8%, positive predictive value 100%, negative predictive value 100%. The two incorrect cases were diagnosed with neuroendocrine tumor and pancreatic cancer upon further examination. There were no procedure-related adverse events [Table 2]. The detailed final diagnosis is summarized in Table 3.
Table 2.
Clinical outcomes and adverse event of EUS-FNB
| Variables | Total (n=75) | |
|---|---|---|
| Technical success, n (%) | 75 (100) | |
| Number of needle pass, n (range) | 2 (2-5) | |
| Two, n (%) | 44 (58.7) | |
| hree, n (%) | 27 (36.0) | |
| Four, n (%) | 3 (4.0) | |
| Five, n (%) | 1 (1.3) | |
| Biopsy | Final diagnosis | |
| Malignant | Benign | |
| Malignant | 29 | 0 |
| Benign | 1* | 44 |
| Inconclusive | 0 | 1* |
| Sensitivity | 96.7% (95% CI: 0.828-0.999) (29/30) | |
| Specificity | 97.8% (95% CI: 0.882-0.999) (44/45) | |
| Positive predictive value | 100% (29/29) | |
| Negative predictive value | 100% (44/44) | |
| Overall accuracy | 97.3% (95% CI: 0.898-0.995) (73/75) | |
| Procedure-related adverse event, n (%) | 0 | |
*Two patients were diagnosed as neuroendocrine tumor and pancreatic cancer, respectively. CI: Confidence interval
Table 3.
Final diagnosis of included patients
| Final diagnosis | n=75 |
|---|---|
| Benign, n | 45 |
| Serous cystic neoplasm | 6 |
| Neuroendocrine tumor | 14 |
| Solid pseudopapillary neoplasm | 6 |
| Reactive lymph node | 2 |
| Adrenal adenoma | 1 |
| Autoimmune pancreatitis, Type 1 | 1 |
| Autoimmune cholangitis | 1 |
| Epidermoid cyst in intrapancreatic accessory spleen | 1 |
| Intraductal papillary mucinous neoplasm | 1 |
| Gastrointestinal stromal tumor | 1 |
| Inflammation or nonpathogenic | 11 |
| Malignant, n | 30 |
| Pancreatic cancer | 20 |
| Cholangiocarcinoma | 3 |
| Gallbladder carcinoma | 2 |
| Lymphoma | 2 |
| Lung cancer | 1 |
| Ampulla of vater cancer | 1 |
| Renal cell cancer | 1 |
DISCUSSION
In this study, we found that MOSE provided a high accuracy of 97.3% with a median of only two or three needle passes in the majority (91.2%) of the patients during the diagnosis for oncologic lesions in abdominal organs. Our results suggest that MOSE may be a viable alternative to ROSE in improving sample acquisition and accuracy for oncologic diagnosis.
Currently, there are no standardized protocols for optimal tissue acquisition by MOSE. Iwashita et al. suggested that MVCs larger than 4 mm can be an indicator of specimen adequacy for improved diagnostic yield;[20] the authors reported that the group with MVCs smaller than 4 mm had an overall sensitivity of 57.1%, whereas the group with MVCs 4 mm or larger showed an overall sensitivity of 95.5% in per pass analysis. In per lesion analysis, the overall sensitivity and accuracy were 94.1% and 95.5%, respectively, regardless of the length of the MVC.[20] As most endosonographers perform at least 2 or more needle passes, the result of the per lesion would be more important, and measuring the length of MVC could be a troublesome process with no significant practical values. In the study by Leung Ki et al., the authors stopped additional needle pass if there was any visible tan-pink core after careful inspection.[21] In our previous report, we divided the patients depending on the quality of visible core on MOSE into (1) definite visible tissue core with scanty blood clots, (2) visible tissue core with moderate blood clots, and (3) scanty tissue core mainly with blood clots.[22] However, the distinction between a visible tissue core and a scanty tissue core is quite vague, and the diagnosis was available even in patients with scanty tissue cores. Notably, our results show that accurate diagnoses could be made with any amount of whitish MVC. Furthermore, using a filter paper helped us better evaluate the specimen by MOSE as it distinguished the whitish MVC more clearly by absorbing the surrounding blood, which often hinders pathologists from making accurate diagnoses. Specimens on filter papers can be fixed with formalin similar to the process for regular endoscopic biopsy specimens, and this also saves time by eliminating the need to make multiple slides as required in ROSE.
There are several limitations to this study. First, all procedures were performed by a highly experienced endosonographer in a high-volume center with an experienced pathology department, and the results of MOSE may somewhat vary in other clinical settings. Second, as we used only one type of needle, the results may be different when using different types of needles. Third, the definition of bloody tissue granule is subjective depending on the endosonographer's experience and some degree of training may be required to distinguish the tissue granules from the bloody samples. Although MVC did not always appear on the specimens, accurate diagnosis was possible when there were bloody tissue granules. Due to retrospective design, we were not able to count precise number of cases with sole bloody tissue granules. Further, well-designed study should be conducted in future. Forth, some cystic lesions are included in the study, but the result of this study should not be generally applied to usual cystic lesions (such as serous cystic neoplasm, intraductal papillary mucinous neoplasm or epidermoid cyst) as there could be selection bias. Finally, this study did not compare the results of MOSE to those of ROSE in a side-by-side manner, so a future study directly comparing the two methods is warranted.
This study has strength in that we analyzed the data of consecutive patients who were prospectively enrolled, and thus the possibility of selection bias would have been reduced. We also included various types of diseases, more than half of which were benign, including cystic lesions. Thus, our results are not limited to suspicious malignant solid masses.
CONCLUSIONS
MOSE with a 22 G needle was critically useful in acquiring tissue samples and producing high diagnostic accuracy, which was possible with small amounts of whitish MVC of bloody tissue granules. Thus, MOSE may be a viable alternative to ROSE in select clinical situations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kandel P, Wallace MB. Recent advancement in EUS-guided fine needle sampling. J Gastroenterol. 2019;54:377–87. doi: 10.1007/s00535-019-01552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oh D, Seo DW, Hong SM, et al. The usefulness of contrast-enhanced harmonic EUS-guided fine-needle aspiration for evaluation of hepatic lesions (with video) Gastrointest Endosc. 2018;88:495–501. doi: 10.1016/j.gie.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Puli SR, Bechtold ML, Buxbaum JL, et al. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass.: A meta-analysis and systematic review? Pancreas. 2013;42:20–6. doi: 10.1097/MPA.0b013e3182546e79. [DOI] [PubMed] [Google Scholar]
- 4.Thornton GD, McPhail MJ, Nayagam S, et al. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: A meta-analysis. Pancreatology. 2013;13:48–57. doi: 10.1016/j.pan.2012.11.313. [DOI] [PubMed] [Google Scholar]
- 5.Sadeghi A, Mohamadnejad M, Islami F, et al. Diagnostic yield of EUS-guided FNA for malignant biliary stricture: A systematic review and meta-analysis. Gastrointest Endosc. 2016;83:290–80. doi: 10.1016/j.gie.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 6.Kim TH. How to improve the diagnostic accuracy of EUS-FNA in abdominal and mediastinal lymphadenopathy? Clin Endosc. 2019;52:93–4. doi: 10.5946/ce.2019.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hébert-Magee S, Bae S, Varadarajulu S, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: A meta-analysis. Cytopathology. 2013;24:159–71. doi: 10.1111/cyt.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matynia AP, Schmidt RL, Barraza G, et al. Impact of rapid on-site evaluation on the adequacy of endoscopic-ultrasound guided fine-needle aspiration of solid pancreatic lesions: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2014;29:697–705. doi: 10.1111/jgh.12431. [DOI] [PubMed] [Google Scholar]
- 9.Kong F, Zhu J, Kong X, et al. Rapid on-site evaluation does not improve endoscopic ultrasound-guided fine needle aspiration adequacy in pancreatic masses: A meta-analysis and systematic review. PLoS One. 2016;11:e0163056. doi: 10.1371/journal.pone.0163056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hewitt MJ, McPhail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest Endosc. 2012;75:319–31. doi: 10.1016/j.gie.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 11.Polkowski M, Jenssen C, Kaye P, et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline-March 2017. Endoscopy. 2017;49:989–1006. doi: 10.1055/s-0043-119219. [DOI] [PubMed] [Google Scholar]
- 12.Wani S, Mullady D, Early DS, et al. The clinical impact of immediate on-site cytopathology evaluation during endoscopic ultrasound-guided fine needle aspiration of pancreatic masses: A prospective multicenter randomized controlled trial. Am J Gastroenterol. 2015;110:1429–39. doi: 10.1038/ajg.2015.262. [DOI] [PubMed] [Google Scholar]
- 13.Kappelle WF, Van Leerdam ME, Schwartz MP, et al. Rapid on-site evaluation during endoscopic ultrasound-guided fine-needle aspiration of lymph nodes does not increase diagnostic yield: A randomized, multicenter trial. Am J Gastroenterol. 2018;113:677–85. doi: 10.1038/s41395-018-0025-8. [DOI] [PubMed] [Google Scholar]
- 14.Van Riet PA, Cahen DL, Poley JW, et al. Mapping international practice patterns in EUS-guided tissue sampling: Outcome of a global survey. Endosc Int Open. 2016;4:E360–70. doi: 10.1055/s-0042-101023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tournoy KG, Praet MM, Van Maele G, et al. Esophageal endoscopic ultrasound with fine-needle aspiration with an on-site cytopathologist: High accuracy for the diagnosis of mediastinal lymphadenopathy. Chest. 2005;128:3004–9. doi: 10.1378/chest.128.4.3004. [DOI] [PubMed] [Google Scholar]
- 16.Eloubeidi MA, Tamhane A, Jhala N, et al. Agreement between rapid onsite and final cytologic interpretations of EUS-guided FNA specimens: Implications for the endosonographer and patient management. Am J Gastroenterol. 2006;101:2841–7. doi: 10.1111/j.1572-0241.2006.00852.x. [DOI] [PubMed] [Google Scholar]
- 17.Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339–49. doi: 10.1055/s-0034-1393354. [DOI] [PubMed] [Google Scholar]
- 18.Aadam AA, Wani S, Amick A, et al. A randomized controlled cross-over trial and cost analysis comparing endoscopic ultrasound fine needle aspiration and fine needle biopsy. Endosc Int Open. 2016;4:E497–505. doi: 10.1055/s-0042-106958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohamadnejad M, Mullady D, Early DS, et al. Increasing number of passes beyond 4 does not increase sensitivity of detection of pancreatic malignancy by endoscopic ultrasound-guided fine-needle aspiration. Clin Gastroenterol Hepatol. 2017;15:1071–8. doi: 10.1016/j.cgh.2016.12.018. e2. [DOI] [PubMed] [Google Scholar]
- 20.Iwashita T, Yasuda I, Mukai T, et al. Macroscopic on-site quality evaluation of biopsy specimens to improve the diagnostic accuracy during EUS-guided FNA using a 19-gauge needle for solid lesions: A single-center prospective pilot study (MOSE study) Gastrointest Endosc. 2015;81:177–85. doi: 10.1016/j.gie.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 21.Leung Ki EL, Lemaistre AI, Fumex F, et al. Macroscopic onsite evaluation using endoscopic ultrasound fine needle biopsy as an alternative to rapid onsite evaluation. Endosc Int Open. 2019;7:E189–94. doi: 10.1055/a-0770-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh D, Seo DW, Hong SM, et al. The impact of macroscopic on-site evaluation using filter paper in EUS-guided fine-needle biopsy. Endosc Ultrasound. 2019;8:342–7. doi: 10.4103/eus.eus_34_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chong CC, Lakhtakia S, Nguyen N, et al. Endoscopic ultrasound-guided tissue acquisition with or without macroscopic on-site evaluation: Randomized controlled trial. Endoscopy. 2020;52:856–63. doi: 10.1055/a-1172-6027. [DOI] [PubMed] [Google Scholar]


