Fig 1.
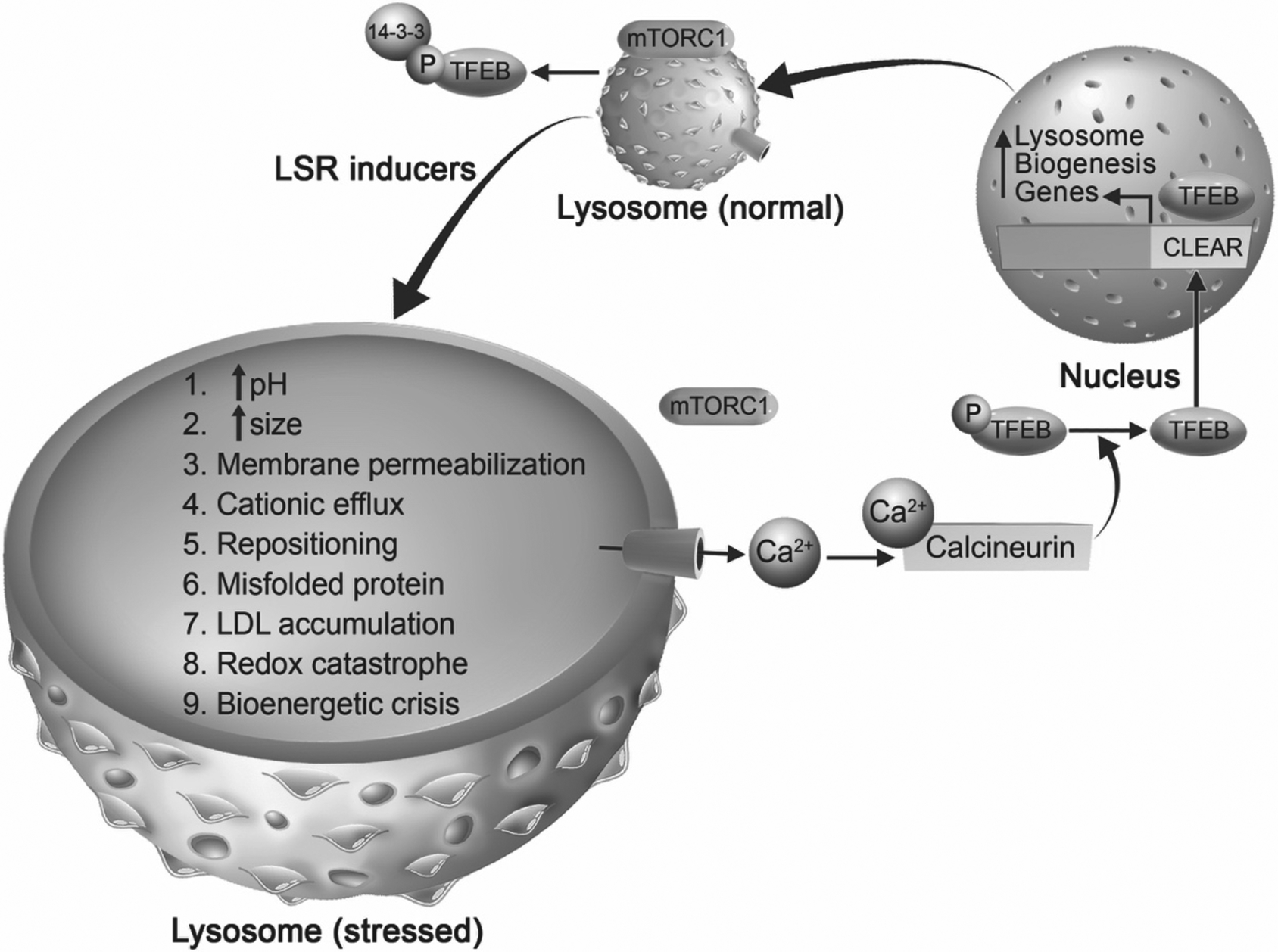
Involvement of mTOR-TFEB in mediating lysosomal stress responses (LSR). Under unstressed conditions, mammalian target of rapamycin complex 1 (mTORC1) is localized on lysosome membranes and phosphorylates transcription factor EB (TFEB), which is a master regulator of lysosome biogenesis. Phosphorylated TFEB is bound by 14-3-3 protein promoting the sequestration of TFEB in the cytoplasm. Under stressful conditions, which can be caused by any drug, pathogen, or material that enters the endolysosome system (termed LSR inducers), lysosomes undergo lysosomal stress responses (labeled 1–9). We posit that during LSR, stressed lysosomes can use various signals, one of which being mTOR-TFEB signaling pathway to promote lysosome biogenesis. When lysosomes are stressed, mTORC1 is released from lysosome membranes, calcium is released through calcium permeable channels (depicted by blue cylinder) into the cytosol where it activates the cytosolic phosphatase calcineurin. Activated calcineurin dephosphorylates TFEB allowing it to translocate into the nucleus where it binds at the coordinated lysosomal expression and regulation (CLEAR) element of lysosome biogenesis genes. Subsequently, there is an increase of lysosome-related genes that lead to the possible restoration of stressed lysosomes. Thus, LSR may use lysosome biogenesis as one of the pathways to restore lysosome function.
