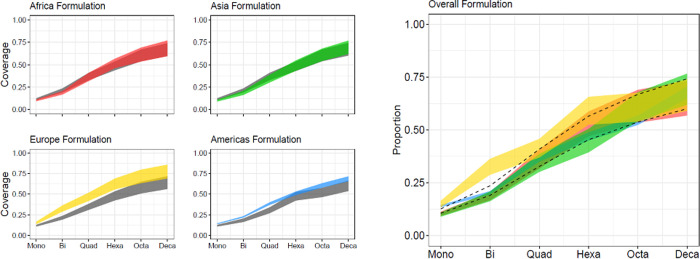
Fig 3.
Estimates of the proportion of C. jejuni isolate coverage for a capsule-conjugate-based vaccine by region and valency using (a) region-specific and (b) global formulations. (a) The x-axis represents the coverage by a stepwise addition of the most prevalent capsule types in each region (mono = monovalent, bi = bivalent, quad = quadrivalent, hexa = hexavalent, octa = octavalent, deca = decavalent) and the y-axis represents the estimated coverage calculated by the pooled prevalence of the proposed vaccine. The shaded region shows the estimated coverage, with the lower bound including non-typable isolates in the pooled prevalence calculations and the upper bound excluding non-typable isolates. The colored area (red = Africa, green = Asia, yellow = Europe, and blue = Americas) represents the coverage achieved by a region-specific formulation (S2 Table). The gray area represents the global coverage with the use of each respective region-specific formula. (b) The x-axis represents the coverage by a stepwise addition of the most prevalent capsule types globally (mono = monovalent, bi = bivalent, quad = quadrivalent, hexa = hexavalent, octa = octavalent, deca = decavalent) and the y-axis represents the estimated coverage calculated by the pooled prevalence of the proposed vaccine. The dotted lines show the estimated coverage of the global vaccine formulation if applied to all regions (globally). The colored regions (red = Africa, blue = Americas, green = Asia, and yellow = Europe) show the estimated coverage of the global vaccine formulation in each individual region (S2 Table). The lower bound represents the estimated coverage including non-typable isolates in the pooled prevalence calculations and the upper bound represents the estimated coverage excluding non-typable isolates.