FIGURE 1.
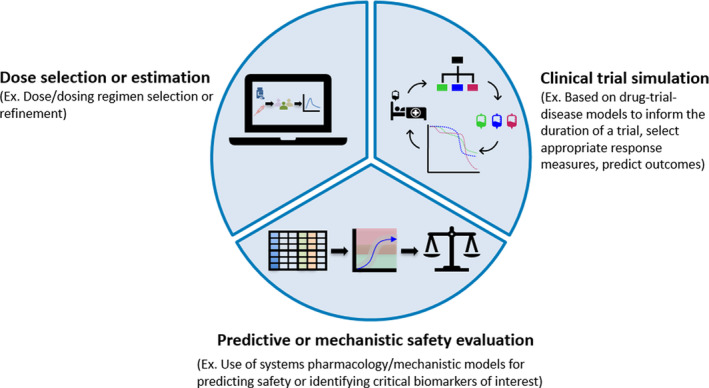
Priority areas for the model‐informed drug development (MIDD) pilot program. As described in the Federal Register Notice, 1 the initial MIDD priority areas include dose selection or estimation, clinical trial simulation, and predictive or mechanistic safety evaluation
