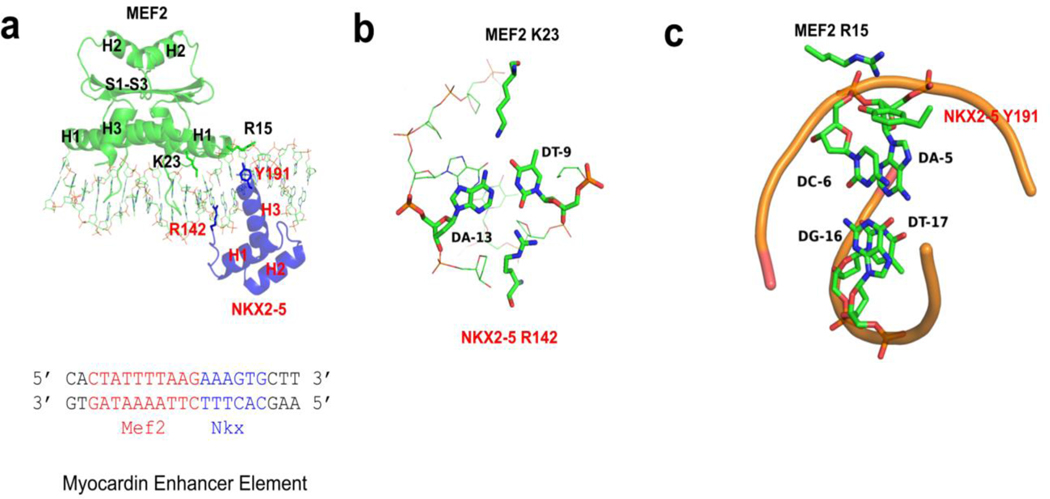
Figure 2. Protein and DNA interaction features of MEF2/NKX2-5/DNA structure.
(a) MEF2 chimera/NKX2-5/DNA ternary complex structure. DNA interacting residues of interests (MEF2 R15 and K23, NKX2-5 R142 and Y191) are shown as sticks. The sequence of the DNA in the crystal is shown below, with the MEF2 binding site colored in red and the NKX2-5 binding site colored in blue. H1-H3: helix 1–3; S1-S3: beta strand 1–3. (b) MEF2 K23 and NKX2-5 R142 interact with the same AT base pair from the major and minor groove, respectively. (c) Cation ion and pi interaction between MEF2 R15 and NKX2-5 Y191. MEF2 R15 interacts with phosphate backbone of A-5’. NKX2-5 Y191 interacts with the bases of A-5’ and C-6’.