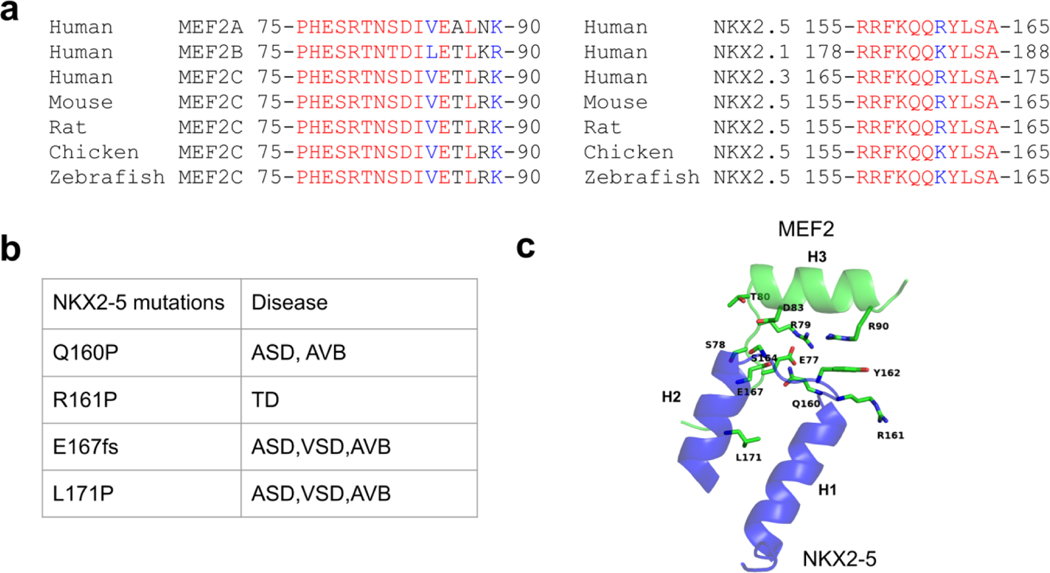
Figure 4. MEF2 and NKX2-5 protein-protein interaction interface is evolutionary conserved.
(a) Sequence alignment of MEF2 and NKX2-5 interface across species. Identical amino acids are colored in red. Amino acids with strong similar properties are colored in blue. (b) NKX2-5 disease related mutations in the interface region in literature. ASD, Atrial Septal Defect; AVB: atrioventricular block; VSD, Ventricular Septal Defect; TD: thyroid dysgenesis. fs: frameshift mutation. (c) Disease relevant residues of NKX2-5 are shown as sticks in the MEF2 and NKX2-5 interaction interface. Residues from MEF2 which are involving interactions with these NKX2-5 residues are shown as sticks and labelled in green. Although NKX2-5 L171 is not involved in direct contact with MEF2, the L171P mutation may impact NKX2-5 and MEF2 interaction as proline is considered to be an alpha helix breaker.