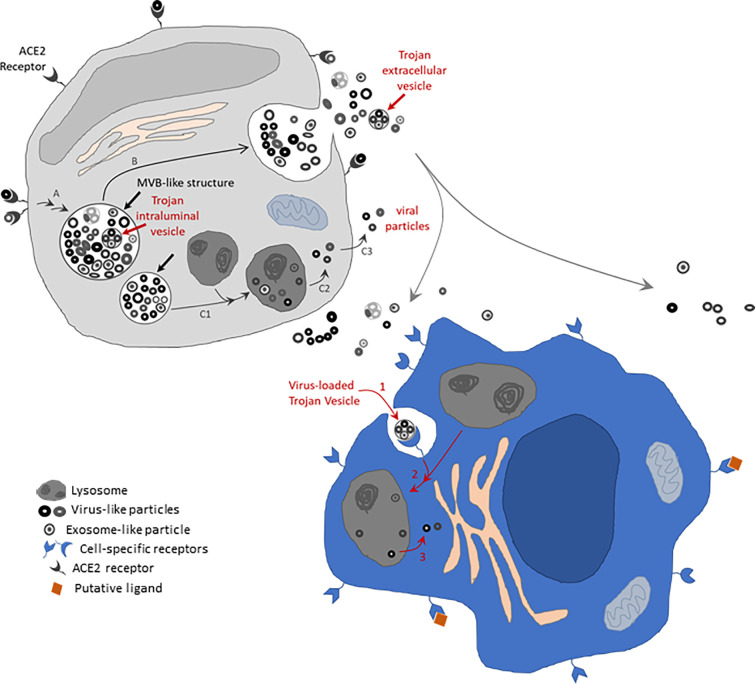
Figure 1.
Schematic of EV-mediated mechanism potentially contributing to SARS-CoV-2 infectivity. Membrane bound structures containing virus-like and exosome-like particles of distinct electron densities and diameters (thick straight black arrows) are shown in the putative grey cell, resembling those structures identified by electron microscopy by Zhu and colleagues (34). These structures resemble multivesicular body (MVB)-like structures. The thick straight red arrow indicates an intraluminal vesicle-like structure that contains smaller nanoparticles [reminiscent of intraluminal vesicles possibly containing hidden viral particles –this also resembles a structure apparently observable in electron micrograph from (34)]. These MVB-like structures might have originated via the endosomal pathway after initial internalization of SARS-CoV-2 particles via the ACE2 receptor in expressing cells (entry route A). The MVB-like structures might proceed to fuse with the cell membrane, therefore spilling their content in the extracellular space (route B). Alternatively, they might fuse with lysosomes and consequently allow scape of the viral particles as suggested by (40) (route C1-C2-C3). Released Trojan extracellular vesicles (EVs) carrying virus-like particles may potentially be internalized by cells not expressing the ACE2 receptor but via cell-specific receptors that recognize specific membrane markers in the Trojan EVs. Internalized Trojan EVs may potentially infect the invaded cells (putative blue cell) by exploiting a mouse hepatitis virus (MHV)-like mechanism (40) via fusion with lysosomes (route 1-2-3 in blue cell). Drawings not done to scale. Membrane proteins expected to be present in viral particles and EVs that are expected to contribute to viral and Trojan EV docking are not drawn. Receptors are drawn as exaggeratedly large features on the cell surface to highlight known (ACE2 receptor-mediated) and potential (Trojan EV receptor-mediated) receptor-specific mechanisms. Trojan EVs might further contribute to SARS-CoV-2 infectivity by transferring ACE2 receptors and other viral molecules to non-expressing cells (not drawn).