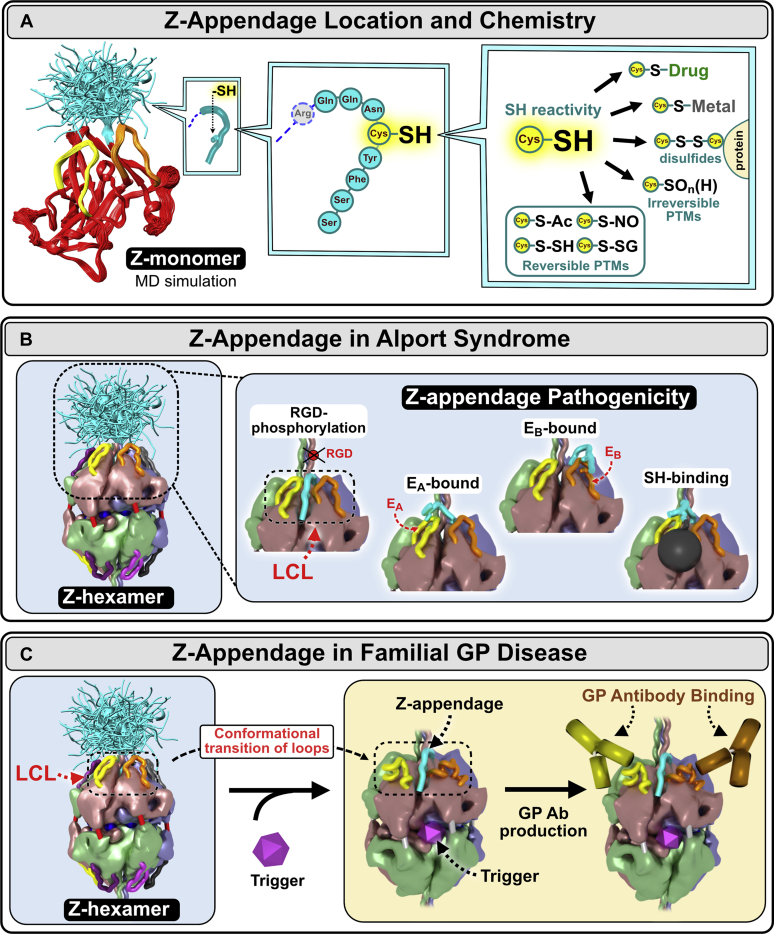
Figure 6.
Z-appendage can participate in multiple chemical interactions and thus can contribute to AS and GP pathogenesis.A, the Z-appendage is an 8-residue extension of the α3 chain replacing the C-terminal histidine residue of the native structure. It is located at the apex of α3NC1 monomer, in juxtaposition with EA and EB hypoepitope loops (left). The appendage is flexible and can assume multiple conformations as predicted by molecular dynamics (MD) simulations (left). Z-appendage features a cysteine residue with free sulfhydryl group (middle). This group can participate in multiple reactions resulting in posttranslational modifications (PTMs), complexes with metal ions or small molecule drugs, and disulfide bonds with proteins (right) (49, 50). B, the appendage has been predicted to assume multiple configurations (left); not shown is the Z-appendage of the opposing α345 trimer. Because of its relatively short length, the expected impact of Z-appendage is on the structure and/or function of a specific area within the α345 hexamer including the EA and EB hypoepitopes and the crevice between them called the loop-crevice-loop (LCL) site. In the pathogenesis of Alport syndrome, Z-appendage can block the RGD integrin binding site (60) or phosphorylation site (61) located in the adjacent triple helical domain, interfere with the EA and EB hypoepitope loops, and attract toxic small molecules and metals to the crevice (inset). C, in Goodpasture’s (GP) disease, Z-appendage can sensitized α345 hexamer, a GP autoantigen, to different second-hit triggers of GP autoantibody production such as environmental toxins or endogenous pathogenic factors, e.g., inflammatory response to bacterial infections, glycoxidative stress in diabetes, etc.