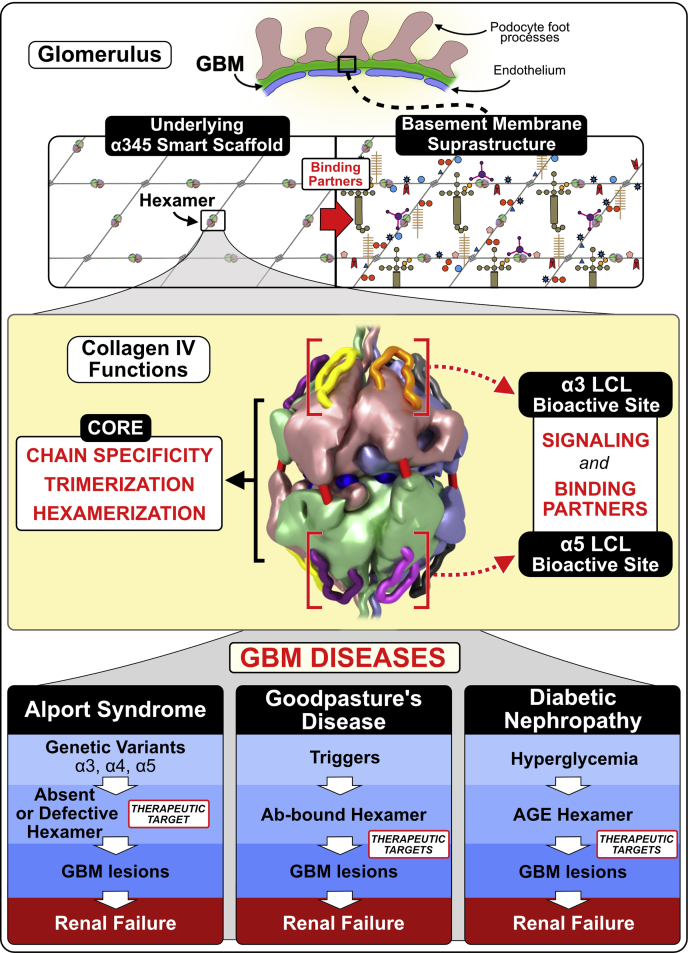
Figure 7.
The α345 hexamer is a focal point in GBM function and dysfunction with LCL sites as potential therapeutic targets in the treatment of GBM kidney diseases. The collagen IVα345 scaffold is a major structure underlying glomerular (GBM) basement membrane. In the GBM, it is deposited by podocytes (62) in the form of protomers that self-assemble in the presence of extracellular levels of chloride ions (63). The scaffold has been defined as a “smart” scaffold (64) due to the presence of multiple binding sites and surfaces that allow participation in quaternary and quinary interactions (65) within the crowded macromolecular environment of the insoluble basement membrane. The α345 hexamer is a key connection module within the collagen IVα345 scaffold. The noncollagenous (NC1) domains of individual α-chains forming the hexamer encode its specific composition and assembly via intracellular trimerization followed by extracellular hexamerization. Quaternary and quinary interactions involving the hexamer surface may include binding partners within the basement membrane and cell surface receptors inducing signaling. There are functional loop-crevice-loop (LCL) bioactive sites at the apices of the hexameric structure (indicated by red square brackets) where pathogenic mechanisms for Alport syndrome, Goodpasture’s disease, and potentially diabetic nephropathy converge. This convergence of pathogenic pathways indicates that the LCL site harbors bioactive functions, including signaling and organizing macromolecular complexes, which underlie the GBM biology. The LCL sites are targets for genetic variants and toxic triggers causing basement membrane abnormalities and leading to renal, pulmonary, otic, and eye disorders. Triggers are envisioned as both environmental and endogenous, e.g., hyperglycemia in diabetes. The LCL sites and downstream pathways are potential targets for rational design of protein replacement and small-molecule therapies. For additional details, see Boudko et al. (49), Fig. 6.